Octet Rule
The octet rule is a useful rule of thumb in chemistry, especially when we visualize chemical bonds using Lewis dot diagrams. Atoms, particularly those with atomic numbers less than 20, may combine in such a way that they attain eight electrons in their valence shells. It's a very stable electron configuration like that of a noble gas. An example is carbon dioxide, shown below in a Lewis dot diagram. Carbon [C] atoms have four valence electrons (1s2 2s2 2p2) and oxygen [O] atoms have six valence electrons (1s2 2s2 2p4). Remember that the inner 1s orbital electrons are not valence electrons. Carbon forms a stable bond by sharing valence electrons with two oxygen atoms, with each bond contributing two electrons to carbon's valence shell, making it a stable octet. Each oxygen atom also forms its own octet:
The shared electrons count in both the oxygen octets and the carbon octet. The total energy of the carbon dioxide molecule is lower than the energy of the three separate atoms involved. Ionic and covalent bonds, two main kinds of bonding, usually follow the octet rule.
BONDING: IONIC VERSUS COVALENT
The first thing to keep in mind is that ionic and covalent bonds aren't mutually exclusive. There are many examples of bonds that fall in between ionic and covalent, forming a spectrum of bonding with covalent at one end and ionic at the other. Pure ionic bonding, in fact, doesn't even exist. There is always at least some covalent nature, some electron sharing in other words, to it. Some elements can form either kind of bond. Hydrogen [H], for example, forms bonds ranging from strongly ionic in nature to strongly covalent. We'll define these bonds as we go on.
Electronegativity: How Atoms Choose Which Kind of Bond
The type of bonding that happens depends on the electronegativity of each atom. Electronegativity is a chemical property that describes the tendency of an atom to attract electrons to itself. It depends on the number of electrons in an atom's valence or outermost shell as well as how far those valence electrons are from the atom's nucleus. This brief two-minute video describes the difference between ionic and covalent bonding:
The screen shot from Wikipedia below shows how electronegativity trends in the periodic table of elements:
The higher the number, the more electronegative the atom is. Sodium [Na] has a relatively low number, 0.93. It tends to form positive ions (Na+), for example, whereas fluorine [F] has the highest number of all, 3.98. It will only form a negative ion when it bonds and it really wants to attract an electron to itself.
The difference in electronegativity between two atoms determines whether they will form an ionic bond or a covalent bond. A larger difference tends toward an ionic bond and a smaller difference or no difference tends toward a covalent bond.
Sodium Chloride: A Typical Ionic Bond
Sodium [Na] and chlorine [Cl] are two atoms with a large difference in electronegativity, 3.16 - 0.93. They react, creating a strong ionic bond to form sodium chloride or table salt.
Sodium, an alkali metal belonging to group 1 on the periodic table, has one more electron than a noble gas, meaning that it has a lone valence electron, while chlorine, a halogen belonging to group 17, is one electron short of a noble gas. Ionic bonding for them is the perfect solution. As you might have guessed, the chlorine and the sodium atoms have a lower energy total state when they bond. That is what drives the bonding reaction. It has to be energetically favourable in order to happen. The reaction Na+ (g) + Cl- (g) → NaCl (s) releases 787 kJ/mol of energy. Likewise, it takes 787 kJ/mol of energy to convert sodium chloride back into its two gas components. Interestingly, it actually takes a little energy to remove an electron from the sodium atom but the accepting of the electron by the chlorine atom releases energy and the attraction of the two ions together lowers the total energy of the system. This energy savings overcomes the smaller amount of energy that must be put into the reaction. For ionic solids such as sodium chloride, the bond energy (787 kJ/mol) is often called lattice energy. It's the total electrostatic potential energy of the lattice formed when these atoms bond with each other. So what is lattice structure all about?
Bonding Determines The Shape Of A Molecule
In ionically bonded materials, many of which are solids arranged in a three-dimensional lattice like sodium chloride, atoms tend to pack themselves in as close as they can to each other. This represents the lowest possible overall energy of the system. Larger chlorine ions pack in as tightly as possible with smaller sodium ions to create a tight regular lattice like this:
The atoms in an ionic lattice are in ion form, with positive cations such as sodium ions and negative anions such as chlorine ions, reflecting the fact that they exchange electrons when they bond.
Covalent bonding is a little different from ionic bonding. It happens when pairs of electrons are shared among atoms, rather than exchanged. It operates on the same general principles as ionic bonding but it achieves its favourable lower energy state a little bit differently.
Covalently bonded atoms attain stable (lowest energy) electron configurations by sharing electrons, rather than through the attraction of oppositely charged ions. Possible molecular shapes, therefore, can be much more variable than they are for ionic molecules. Organic chemistry (the study of carbon-based compounds) and stereochemistry (the science of molecular shapes) focus on covalent bonding. Consider the complex shape of a protein molecule, for example, shown below. It is an organic molecule. The peptide bond is an example of a covalent bond and it is highlighted in the box:
(Chemistry-grad-student; Wikipedia)
Covalent Bonding: Another Way to Lower Energy
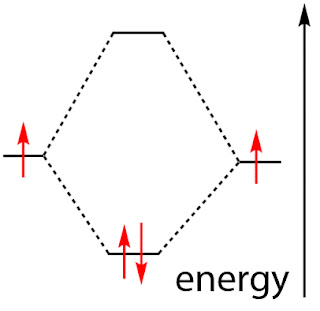
Covalent bonding is possible because electrons can pair up. They do this for the same basic reason that ionic bonds attempt to fill and stabilize orbitals - to find a lower-energy, more stable state. Like ionic bonding, electron pairing happens in the valence shell of each atom. Electrons in the same orbital must have opposite spins, but they're both negatively charged too, and they tend to repel each other. This repulsion is overcome by the lower energy state that pairing up can offer. In the diagram below, called a molecular orbital (MO) diagram, electron spins are shown as red arrows. Two unpaired electrons, with the same spins, represent a considerably higher energy state than two paired (opposite spin) electrons, shown below.
This pair can be a lone pair, not involved in chemical bonding, or a bonding pair. Covalent bonds can be created with single or multiple electron pairs, in the latter case forming double and triple bonds. Double bonds do not change the overall shape of the molecule but they are stronger than single bonds. Triple bonds are stronger yet. Remember that covalent bonding is simply a more even sharing of electrons between two atoms than ionic bonding. It is favoured when the electronegativity of the two atoms is similar or identical (as when two atoms of the same element bond together).
Carbon-Carbon Bonds are Typical Covalent Bonds
Let's examine a double bond between two carbon atoms. These bonds are often found in organic molecules. We'll take ethylene (C2H4) as our example to get an idea of how it works. It is drawn as a Lewis dot diagram below:
Carbon atoms have an orbital configuration of 1s22s22p2. These atoms have four valence electrons, two 2s electrons and two 2p electrons, each one of which is available for bonding. Both the 2p and 2s orbitals are valence orbitals for carbon atoms. When carbon bonds with another atom, it has a fascinating ability to modify its orbitals to "fit together" with another atom's orbitals. The "fitting together" is actually the sharing of orbitals, which we'll explore next in Atoms Part 4C: Bond Hybridization.






No comments:
Post a Comment
Note: Only a member of this blog may post a comment.