We live under spectacular skies:
The sky, the air we breathe, is called the atmosphere. It is a very thin layer of gases that surrounds our rocky planet. Astronauts onboard the International Space Station took this spectacular image of the atmosphere as seen from orbit, over the Indian Ocean at sunset. You can just make out the curvature of Earth as a slight arc. Different layers of the atmosphere show up as different colours.
The gases in our atmosphere make up a layer that is approximately 100 km thick, of which only the deepest layer can sustain life. In fact, many of us have trouble breathing at altitudes over 3000 m above sea level. That isn't even as high up as Mount Everest's base camp! At high altitudes, oxygen becomes scarce and air pressure is very low. You have to breathe faster and deeper to get enough oxygen to breathe. This problem is compounded by the increasing scarcity of carbon dioxide at altitude, another essential atmospheric gas. Without carbon dioxide accumulation in the lungs, our bodies aren't signaled to breathe harder. Under these conditions, we can suffer from altitude sickness, which can range from a series of mild symptoms such as headache and lightheadedness to severe and life-threatening pulmonary and cerebral edema (increased fluid in, and swelling of, the tissues).
This extremely thin, fragile and vital layer of gases surrounding Earth sustains and protects life. If Earth were the size of an apple, its atmosphere would be less than the thickness of its peel. It has gone through dramatic changes over the eons and yet somehow life has managed to evolve and spread across its entire surface. As scientists study the atmospheres of other planets, and begin to look at the atmospheres of exoplanets, planets outside our solar system, we are begining to appreciate just how rare and precious our air is. Earth's atmosphere insulates the surface from extreme temperatures, it keeps sufficient heat inside and it blocks the surface from the Sun's deadly radiation. There would be no possibility of life, as we know it, without the protection of our atmosphere.
This 5-minute National Geographic video provides a good introduction to Earth's atmosphere:
In this atmosphere series of articles, we will explore what our atmosphere is made up of and how it is structured, how weather is made, how our climate tends to stay in balance as well as how it has changed over time. We will learn how our atmosphere sustains life and what makes it special compared to the atmospheres of other planets. Finally we tackle global warming - is it real? Are our activities changing our atmosphere, and can we live with those changes?
Each of us breathes on average about 10,000 litres of air every day, without thinking much about it. We live within a highly complex system of plants and animals, all of which hinges upon our breathable atmosphere. Let's look at what it's made of, next, in Earth's Atmosphere Part 2.
Monday, January 30, 2012
Earth's Atmosphere Part 1 - Introduction
Labels:
Atmosphere Series
Sunday, January 29, 2012
Earth's Atmosphere Part 2 - Composition
Earth's atmosphere is composed of a wide variety of gases, almost all of which play essential roles in maintaining life. Many of these gases cycle nutrients and energy through Earth's biosphere, the outer layer of Earth which contains all life, from birds, to fish to land-dwellers like us, making these nutrients and energy available to us. Our lives, and the lives of all organisms, are inextricably tied to the gases of our atmosphere.
This pie chart shows the relative amounts of these gases:
Nitrogen
Most of Earth's atmosphere is composed of nitrogen gas (78%), a colourless odourless and mostly inert, or nonreactive, gas. Although we do not need nitrogen to breathe, it is essential for life on Earth. The nitrogen cycle takes mostly unusable nitrogen gas from the air and stabilizes it in compounds that plants use for photosynthesis and growth. This vital job, called nitrogen fixation, is carried out by special bacteria in the soil.
Plants are the foundation of almost every one of Earth's food webs. They provide food for all other organisms. If you are unsure what a food web is, take a look at this 3-minute video:
We obtain the nitrogen we need by eating plants and other animals. It makes up a significant part of our bodies, as part of the building blocks of our proteins and nucleic acids.
Nitrogen's Dark Side: The Bends
We breathe in (mostly) nitrogen with every breath and it is harmless. Deep sea divers, on the other hand, know that it can be potentially fatal under certain circumstances. At depths of about 30 metres or more, the pressure of the air breathed from a SCUBA tank is about four times higher than air pressure on land. It has to have the same pressure as the surrounding water, or air would not come out of the tank. Normally no nitrogen will dissolve into the blood in your lungs, but when it is under pressure at this depth, some nitrogen will pass from the air and dissolve in your blood. This is the same principle behind making carbonated sodas. The soda is exposed to carbon dioxide gas under pressure and carbon dioxide becomes dissolved in the drink. Under a tight cap, it remains dissolved but once the cap is removed and the pressure is released, the soda fizzes as carbon dioxide escapes in tiny bubbles. If a SCUBA diver ascends too quickly, the nitrogen gas dissolved in his blood will, just like the soda, escape out of his blood into his body tissues, especially, and very painfully, into joints, hence the name "the bends." Bubbles of gas in the bloodstream are always dangerous because they can potentially block the blood flow in an artery and precipitate a stroke or heart attack. The bends, or decompression sickness as it is technically called, can be prevented by slowing ascension enough to prevent the excess formation of nitrogen bubbles. Another way to prevent the bends is to use a gas mixture with a different formulation than ordinary air, a common one being Nitrox, air enriched with up to 36% oxygen, thus reducing the amount of nitrogen present and thereby reducing the possibility of the bends, shown here:
This mixture comes with its own risk, however. If it is used below the maximum recommended depth (28 metres for 36%), the increased oxygen level can lead to oxygen toxicity, a dangerous condition we will explore a bit later.
Oxygen
21% of our atmosphere is made up of oxygen, a very pale blue odourless gas. Unlike nitrogen, oxygen is highly reactive and easily forms compounds with other elements. Although oxygen is very abundant as an element (it makes up almost half of Earth's crust by weight), it is too reactive to exist for long as either a molecular (O2) or an atomic (O) gas in our atmosphere. Its level is maintained through the photosynthesis of billions of plants, all pumping out oxygen gas as a by-product of a reaction between carbon dioxide and water to create sugars that plants use for energy and growth. The reactions involved in photosynthesis take place inside chloroplasts, special organelles within plant cells:
Every green part of every plant contains chloroplasts that are filled with green pigments called chlorophyll. Chlorophyll, one of Nature's most brilliant inventions, absorbs sunlight in the blue and red parts of the spectrum and transfers that energy to the what is called the reaction centre of the chloroplast, a complex of pigments, proteins and enzymes that turn sunlight into the chemical energy that is stored in sugar molecules.
If all the plants on Earth suddenly disappeared, oxygen gas would eventually be reabsorbed into Earth's rocky crust through the chemical process of oxidation. The ten most common compounds in Earth's crust are all oxides, such as silicon dioxide and iron oxide for example, in which oxygen is chemically bound up. Some experts estimate it would take only about 5000 years to completely resorb all of Earth's atmospheric oxygen.
We, and all respiring organisms, need oxygen to live. When we breathe in air, alveoli in our lungs exchange carbon dioxide for oxygen, as shown here in this diagram:
Our red blood cells transport oxygen to our cells where it reacts with glucose (an energy-rich sugar) to create ATP (adenosine triphosphate). ATP is an incredibly efficient energy storage molecule that drives all metabolic processes of life, from the simplest bacteria to plants to animals, with water and carbon dioxide as by-products. Almost all animals, from simple unicellular protozoa to humans, use oxygen in this process, called cellular respiration. Some very primitive organisms, however, live in oxygen-free environments and obtain their energy from fermentation or chemical reactions that use sulfates, nitrates or sulfur as an oxidizing agent rather than oxygen. Many of these simple organisms play essential roles in the nitrogen, sulfur and carbon cycles that make life possible on Earth.
Although oxygen is essential to almost all life on Earth, there is such a thing as too much of a good thing. Too much oxygen, a concern for SCUBA divers, and a possibility for those on high supplemental oxygen (usually O2 under pressure) such as premature babies and people undergoing hyperbaric oxygen therapy, can lead to cell damage and death, with symptoms most often developing in the central nervous system, lungs and eyes. This damage occurs through a condition called oxidative damage, in which too much oxygen in cells overcomes their ability to detoxify the highly reactive intermediate molecules produced during metabolic activities. Too many peroxides and free radicals, for example, can soon overcome a cell and damage or kill it. Oxidative stress underlies some of the damage associated with many diseases including atherosclerosis and heart failure. Under strictly controlled conditions, however, reactive oxygen-based molecules can be beneficial. Our immune systems use them to kill off pathogens.
Argon
Argon, making up about 1% of the atmosphere, is a colourless odourless noble gas and that means that it is almost completely nonreactive. Its name derives from a Greek word that means "lazy one." Because it is so nonreactive it makes an efficient thermal insulation material between glass panes in energy efficient windows. It is also used to put out fires at web server farms, where it will not damage sensitive electronic equipment. Argon does not take part in any known biological processes on Earth.
Carbon Dioxide
Carbon dioxide (CO2) is so familiar to us, both as a natural byproduct of respiration and as a much-maligned pollutant and greenhouse gas, that you might be surprised to learn that it comprises just a mere 0.04% of our atmosphere.
It is part of the carbon cycle and of photosynthesis, two processes essential to life on Earth.
The basic carbon cycle is illustrated in this diagram:
Of all the carbon dioxide emitted into the atmosphere, one quarter is taken up by plants and another quarter is taken up by oceans. Plants, as well as two simple but very abundant organisms - algae and cyanobacteria - absorb carbon dioxide from the air and bind it into sugars and carbohydrates. Animals produce carbon dioxide and release it into the atmosphere. Volcanoes, hot springs and geysers and carbonate rocks also contribute to atmospheric carbon dioxide. Fires and combustion reactions in engines release the carbon dioxide bound up in plant material back into the atmosphere as well. Over the short term (year by year), carbon dioxide levels tend to stay within a fairly narrow range of atmospheric concentrations, fluctuating naturally with the seasons, decreasing slightly in the spring and summer and increasing in winter, based on changes in photosynthesis and respiration.
However, carbon dioxide naturally increases as global temperatures increase, forming a positive feedback loop. A large part of this loop takes place in the oceans. The oceans are (at present) gigantic carbon sinks, which means that they absorb carbon dioxide out of the atmosphere, where it is dissolved in water as well as taken up in bicarbonate compounds. As ocean temperature rises, carbon dioxide solubility decreases, so as oceans warm, they absorb less of the gas from the atmosphere. Carbon dioxide is a greenhouse gas. This means that it traps the heat from the Sun in the atmosphere. So, as carbon dioxide builds up in the atmosphere, temperatures rise, the oceans warm, and yet more carbon dioxide is released into the atmosphere. This is the nature of a positive feedback loop, and it is an important facet of the current threat of global warming. There are other positive feedback loops at play in global warming, and we will explore them in detail in a later atmosphere article. What concerns climatologists most about feedback loops like this one is that they tend to take a system out of equilibrium and toward extremes. Life depends on the finely tuned homeostasis of our climate.
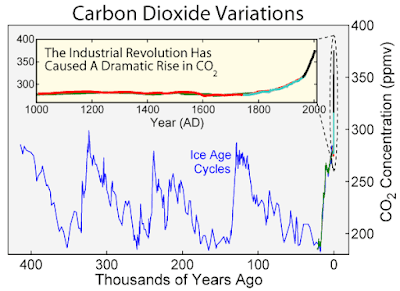
Atmospheric levels of carbon dioxide are carefully monitored because they are have been closely linked with past and present global warming events, and because Earth is currently experiencing an unprecedented spike in atmospheric CO2 levels, concurrent with industrial activity, as this graph shows:
Each of us produces about a kilogram of carbon dioxide every day. For us and all other animals, it is a waste gas. Yet, carbon dioxide also has an important role to play in our physiology. We have special carbon dioxide receptors in our circulatory system. If levels are high, the capillaries, tiny blood vessels where gas exchange in our tissues occurs, expand an allow more blood to the tissue. Breathing, too, is stimulated by high carbon dioxide levels, rather than by oxygen levels, in the blood. That is why low pressure air does not trigger air hunger. This is always a danger for high altitude fighter pilots and why the stewardess always tells us to put on our air mask first in an emergency, because we would not know we are running out of oxygen until we passed out. There is currently some research that links SIDS (Sudden Infant Death Syndrome) with a defect in our CO2 respiration regulation system.
Trace Gases
Our atmosphere also contains on average about 1 - 4 % water vapour. Water vapour, like carbon dioxide, is a major greenhouse gas. It too operates through a positive feedback loop. Increasing water vapour levels contribute to warmer temperatures and warmer temperatures lead to higher water vapour levels. Water vapour has also been shown to amplify the greenhouse effect of other gases. Recent research suggests that atmospheric water vapour could double the warming effect of carbon dioxide.
Other trace gases, such as neon, helium, methane, krypton, hydrogen, nitrous oxide, carbon monoxide, nitrogen dioxide, ozone, xenon, sulfur dioxide and ammonia, also contribute to Earth's atmosphere. Any one of these gases makes up far less than 1% of our atmosphere. All of them have natural origins. Nitrogen, oxygen, carbon dioxide, methane are replenished through biological activity. Hydrogen, sulfur dioxide and ozone are replenished through photochemical reactions in our atmosphere. Some gases are replenished through radioactivity - argon and helium, for example. And some gases, such as xenon, neon and krypton, are expelled from deep within Earth's interior through volcanic activity.
This graphic gives you an idea of the relative amounts of gases in Earth's atmosphere:
Percentages are expressed as composition by volume; ppm means parts per million.
Our atmosphere also contains traces of gases that come from industrial activity. Carbon dioxide, nitrous oxides, carbon monoxide and sulphur dioxide, in addition to natural sources, also come from man-made combustion reactions.
Carbon Monoxide Poisoning
You have probably heard of the dangers of running your car in your garage with the doors closed, or perhaps you have heard of whole families tragically killed by a faulty furnace. The killer is carbon monoxide (CO). This colourless odourless tasteless and extremely poisonous gas is very difficult to detect and so carbon monoxide detectors are recommended for every home. CO is a product of incomplete combustion, usually linked with insufficient oxygen (an enclosed space for example). In the presence of oxygen, carbon monoxide burns with a blue flame, producing carbon dioxide. This is why gas inspectors will check the flame of a gas stove when it is installed. Blue is good. Carbon monoxide binds with hemoglobin, preferentially over oxygen, so that the body cannot deliver oxygen to its cells. Air concentrations as low as 667 parts per million, will cause half of the body's hemoglobin to bind to carbon monoxide rather than oxygen. The body's cells asphyxiate, with symptoms ranging from a mild headache to death. Quick removal of CO from the air (opening the windows and doors or getting outside) can stop the deadly process and save lives. Removal to an environment with sufficient oxygen levels will allow the CO binding process to reverse itself. Follow-up treatment for severe cases is high-dose oxygen therapy in a hospital, where binding reversal can be hastened, to help avoid permanent damage, such as irreversible coma.
Ozone Pollution
It is important to distinguish stratospheric ozone (often called "good" ozone, which we will discuss shortly) from ozone in the lower atmosphere, that is, from the surface up to an altitude of about 2 kilometres. This surface ozone is created by industrial activity and it is a pollutant. It is used as a bleach, a deodorizer and a sterilizer and it is toxic. It is also a product of internal combustion engines and power plants. Nitrous oxides and volatile organic compounds from these activities react with oxygen, especially on hot sunny days, and form ozone as a result. It is a very corrosive gas that can damage the alveoli in your lungs, leading to respiratory infections and inflamed tissues, and aggravating conditions such as asthma. It can also destroy crops and forest vegetation. Some of the highest recorded ozone levels have occurred right here in Canada, in the Toronto area. Much of our ozone pollution drifts up from the U.S. industrial belt. This graphic shows afternoon ground-level ozone concentration for last July:
The yellow line indicates the atmospheric concentration of ozone in Dobson units. Most atmospheric ozone exists in the stratosphere, between the tropopause and stratopause. The ozone concentrations shown are very small, on the order of a few molecules of O3 per million molecules of air. These few molecules are vitally important in blocking out UV radiation, which is divided into three different kinds based on wavelength of the radiation. UV-C, the most deadly, is completely blocked out by the ozone layer and a significant amount of UV-B, the UV radiation strongly linked to sunburn and skin cancer, is blacked out as well.
The ozone layer is replenished naturally through oxygen (O2) reactions. The chlorine ion in chlorofluorocarbons is released in the atmosphere by a photo-induced fission of the Cl-C bond. This ion catalyzes the conversion reaction of ozone into oxygen molecules. Ozone (O3) absorbs UV radiation much better than oxygen (O2) does, so this is why the growing holes in our ozone layer, centered about the two poles, is dangerous to life. The use of chlorofluorocarbons has been almost completely phased out as this writing (2012). Chlorofluorocarbons break down quite readily in the atmosphere. And if they are released lower in the atmosphere they break up without doing damage to the ozone layer. Just the same, the breakdown of stratospheric chlorofluorocarbons has led to much higher amounts of chlorine ions than originally predicted, and these ions remain stable in the atmosphere as they continue to weaken the ozone layer. Ozone depletion has been linked to increased rates of skin cancer, cataracts, damage to plants and reduction in plankton populations, particularly at latitudes over 35°.
You might have heard of other gases in the media that are being closely monitored for their potential link to global warming, such as methane, carbon dioxide and nitrous oxides. These are all greenhouse gases. They tend to absorb heat from the Sun and trap it in our atmosphere, much like greenhouse glass does. We will explore their potential threat in a future atmosphere article focusing on global warming and climate change.
Acid Rain
Rain is naturally mildly acidic. Carbon dioxide in the air reacts with water to form weak carbonic acid. The threat of acid rain you have probably heard about is an enhanced effect, a result of some of the gases emitted when fossil fuels are burned, particularly sulphur dioxide and nitrous oxides. Fossil fuels often contain sulphur impurities that burn and create sulphur dioxide. Sulphur dioxide reacts with water to create a weak solution of sulphuric acid. Usually, nitrogen and oxygen don't react with each other in the atmosphere. But at very high temperatures, in an engine for example, a small proportion of each gas does react, to create nitrogen oxides. These oxides react with water to create a weak solution of nitric acid. When fresh water pH drops below 5, most fish eggs won't hatch and adult fish begin to die off. Aquatic insects, as well, take a severe hit, reducing the biodiversity in the freshwater ecosystem, thereby weakening it. These systems are affected not only directly by acidic rain but by acidic runoff as well. In soils, some microbes cannot handle increased acidity. Their enzymes, essential for carrying out their metabolic functions, become denatured and they die. As well, toxic metals like aluminum are released from compounds and important minerals such as magnesium and calcium are leached away from soils when pH is lowered. Crops can be fertilized and limed to counteract increasing acidity but plants in the wilderness such as natural forests suffer from the lack of nutrients that results. Humans are generally not directly affected by acid rain but our buildings are. Limestone and marble, which contain lots of calcium carbonate, erode away under acidic conditions. This is why the inscriptions on old gravestones eventually become illegible. Metals, especially iron, steel, copper and bronze, also corrode faster under acidic conditions.
Why Is The Sky Blue?
You might think that the sky is blue because of the contribution of faintly blue oxygen gas, mentioned earlier. This is what liquid oxygen looks like, to give you an idea of its colour:
The sky is blue, not because of oxygen but because of how sunlight is scattered by various gas molecules in Earth's atmosphere. Sunlight is made up of all the colours of the rainbow. When all these colours are combined, they make up pure white light. Prisms (or raindrops suspended in the sky) separate white sunlight into its colours.
Notice that the blue and violet light bends more and is made up of shorter wavelengths. This light has more energy than longer wavelength red light.
When sunlight reaches Earth's atmosphere, gas molecules scatter it in all directions. Higher energy (blue) sunlight tends to scatter more than lower energy (red) sunlight. This process is called Rayleigh scattering. The sky looks blue because we see many of these scattered blue rays. Rayleigh scattering gives Earth a blue halo that is even visible from outer space, as shown in this photo taken by the International Space Station. The moon as a crescent is visible through the blue haze.:
When the Sun gets low on the horizon, sunlight must pass through even more air before it reaches our eyes. Blue light rays are scattered and rescattered many times in many directions. The surface of the Earth has also reflected and scattered the sunlight. As a result, we tend to see more white light and less blue light. As the Sun sets, even more blue light is scattered while longer wave reds and yellows are less scattered, so more of these coloured rays can travel straight to your eyes. Particulate pollution, dust and water vapour all scatter light and contribute to more dramatically colourful sunsets, such as this one in South Africa:
Now that we are familiar with the components that make up Earth's atmosphere, let's explore its structure, next, in Earth's Atmosphere Part 3.
This pie chart shows the relative amounts of these gases:
(Image courtesy ux1.eiu.edu)
Nitrogen
Most of Earth's atmosphere is composed of nitrogen gas (78%), a colourless odourless and mostly inert, or nonreactive, gas. Although we do not need nitrogen to breathe, it is essential for life on Earth. The nitrogen cycle takes mostly unusable nitrogen gas from the air and stabilizes it in compounds that plants use for photosynthesis and growth. This vital job, called nitrogen fixation, is carried out by special bacteria in the soil.
(image courtesy of EPA, Environmental Protection Agency, U.S.)
Plants are the foundation of almost every one of Earth's food webs. They provide food for all other organisms. If you are unsure what a food web is, take a look at this 3-minute video:
We obtain the nitrogen we need by eating plants and other animals. It makes up a significant part of our bodies, as part of the building blocks of our proteins and nucleic acids.
Nitrogen's Dark Side: The Bends
We breathe in (mostly) nitrogen with every breath and it is harmless. Deep sea divers, on the other hand, know that it can be potentially fatal under certain circumstances. At depths of about 30 metres or more, the pressure of the air breathed from a SCUBA tank is about four times higher than air pressure on land. It has to have the same pressure as the surrounding water, or air would not come out of the tank. Normally no nitrogen will dissolve into the blood in your lungs, but when it is under pressure at this depth, some nitrogen will pass from the air and dissolve in your blood. This is the same principle behind making carbonated sodas. The soda is exposed to carbon dioxide gas under pressure and carbon dioxide becomes dissolved in the drink. Under a tight cap, it remains dissolved but once the cap is removed and the pressure is released, the soda fizzes as carbon dioxide escapes in tiny bubbles. If a SCUBA diver ascends too quickly, the nitrogen gas dissolved in his blood will, just like the soda, escape out of his blood into his body tissues, especially, and very painfully, into joints, hence the name "the bends." Bubbles of gas in the bloodstream are always dangerous because they can potentially block the blood flow in an artery and precipitate a stroke or heart attack. The bends, or decompression sickness as it is technically called, can be prevented by slowing ascension enough to prevent the excess formation of nitrogen bubbles. Another way to prevent the bends is to use a gas mixture with a different formulation than ordinary air, a common one being Nitrox, air enriched with up to 36% oxygen, thus reducing the amount of nitrogen present and thereby reducing the possibility of the bends, shown here:
This mixture comes with its own risk, however. If it is used below the maximum recommended depth (28 metres for 36%), the increased oxygen level can lead to oxygen toxicity, a dangerous condition we will explore a bit later.
Oxygen
21% of our atmosphere is made up of oxygen, a very pale blue odourless gas. Unlike nitrogen, oxygen is highly reactive and easily forms compounds with other elements. Although oxygen is very abundant as an element (it makes up almost half of Earth's crust by weight), it is too reactive to exist for long as either a molecular (O2) or an atomic (O) gas in our atmosphere. Its level is maintained through the photosynthesis of billions of plants, all pumping out oxygen gas as a by-product of a reaction between carbon dioxide and water to create sugars that plants use for energy and growth. The reactions involved in photosynthesis take place inside chloroplasts, special organelles within plant cells:
(Image courtesy terra.dadeschools.net)
Every green part of every plant contains chloroplasts that are filled with green pigments called chlorophyll. Chlorophyll, one of Nature's most brilliant inventions, absorbs sunlight in the blue and red parts of the spectrum and transfers that energy to the what is called the reaction centre of the chloroplast, a complex of pigments, proteins and enzymes that turn sunlight into the chemical energy that is stored in sugar molecules.
If all the plants on Earth suddenly disappeared, oxygen gas would eventually be reabsorbed into Earth's rocky crust through the chemical process of oxidation. The ten most common compounds in Earth's crust are all oxides, such as silicon dioxide and iron oxide for example, in which oxygen is chemically bound up. Some experts estimate it would take only about 5000 years to completely resorb all of Earth's atmospheric oxygen.
We, and all respiring organisms, need oxygen to live. When we breathe in air, alveoli in our lungs exchange carbon dioxide for oxygen, as shown here in this diagram:
(Copyright: helix84(Wikipedia)
Our red blood cells transport oxygen to our cells where it reacts with glucose (an energy-rich sugar) to create ATP (adenosine triphosphate). ATP is an incredibly efficient energy storage molecule that drives all metabolic processes of life, from the simplest bacteria to plants to animals, with water and carbon dioxide as by-products. Almost all animals, from simple unicellular protozoa to humans, use oxygen in this process, called cellular respiration. Some very primitive organisms, however, live in oxygen-free environments and obtain their energy from fermentation or chemical reactions that use sulfates, nitrates or sulfur as an oxidizing agent rather than oxygen. Many of these simple organisms play essential roles in the nitrogen, sulfur and carbon cycles that make life possible on Earth.
Although oxygen is essential to almost all life on Earth, there is such a thing as too much of a good thing. Too much oxygen, a concern for SCUBA divers, and a possibility for those on high supplemental oxygen (usually O2 under pressure) such as premature babies and people undergoing hyperbaric oxygen therapy, can lead to cell damage and death, with symptoms most often developing in the central nervous system, lungs and eyes. This damage occurs through a condition called oxidative damage, in which too much oxygen in cells overcomes their ability to detoxify the highly reactive intermediate molecules produced during metabolic activities. Too many peroxides and free radicals, for example, can soon overcome a cell and damage or kill it. Oxidative stress underlies some of the damage associated with many diseases including atherosclerosis and heart failure. Under strictly controlled conditions, however, reactive oxygen-based molecules can be beneficial. Our immune systems use them to kill off pathogens.
Argon
Argon, making up about 1% of the atmosphere, is a colourless odourless noble gas and that means that it is almost completely nonreactive. Its name derives from a Greek word that means "lazy one." Because it is so nonreactive it makes an efficient thermal insulation material between glass panes in energy efficient windows. It is also used to put out fires at web server farms, where it will not damage sensitive electronic equipment. Argon does not take part in any known biological processes on Earth.
Carbon Dioxide
Carbon dioxide (CO2) is so familiar to us, both as a natural byproduct of respiration and as a much-maligned pollutant and greenhouse gas, that you might be surprised to learn that it comprises just a mere 0.04% of our atmosphere.
It is part of the carbon cycle and of photosynthesis, two processes essential to life on Earth.
The basic carbon cycle is illustrated in this diagram:
(Copyright:physicalgeography.net)
However, carbon dioxide naturally increases as global temperatures increase, forming a positive feedback loop. A large part of this loop takes place in the oceans. The oceans are (at present) gigantic carbon sinks, which means that they absorb carbon dioxide out of the atmosphere, where it is dissolved in water as well as taken up in bicarbonate compounds. As ocean temperature rises, carbon dioxide solubility decreases, so as oceans warm, they absorb less of the gas from the atmosphere. Carbon dioxide is a greenhouse gas. This means that it traps the heat from the Sun in the atmosphere. So, as carbon dioxide builds up in the atmosphere, temperatures rise, the oceans warm, and yet more carbon dioxide is released into the atmosphere. This is the nature of a positive feedback loop, and it is an important facet of the current threat of global warming. There are other positive feedback loops at play in global warming, and we will explore them in detail in a later atmosphere article. What concerns climatologists most about feedback loops like this one is that they tend to take a system out of equilibrium and toward extremes. Life depends on the finely tuned homeostasis of our climate.
Atmospheric levels of carbon dioxide are carefully monitored because they are have been closely linked with past and present global warming events, and because Earth is currently experiencing an unprecedented spike in atmospheric CO2 levels, concurrent with industrial activity, as this graph shows:
(This figure was prepared by Robert A. Rohde from publicly available data and is incorporated into the Global Warming Art project and is subject to his copyright (Wikipedia))
Each of us produces about a kilogram of carbon dioxide every day. For us and all other animals, it is a waste gas. Yet, carbon dioxide also has an important role to play in our physiology. We have special carbon dioxide receptors in our circulatory system. If levels are high, the capillaries, tiny blood vessels where gas exchange in our tissues occurs, expand an allow more blood to the tissue. Breathing, too, is stimulated by high carbon dioxide levels, rather than by oxygen levels, in the blood. That is why low pressure air does not trigger air hunger. This is always a danger for high altitude fighter pilots and why the stewardess always tells us to put on our air mask first in an emergency, because we would not know we are running out of oxygen until we passed out. There is currently some research that links SIDS (Sudden Infant Death Syndrome) with a defect in our CO2 respiration regulation system.
Trace Gases
Our atmosphere also contains on average about 1 - 4 % water vapour. Water vapour, like carbon dioxide, is a major greenhouse gas. It too operates through a positive feedback loop. Increasing water vapour levels contribute to warmer temperatures and warmer temperatures lead to higher water vapour levels. Water vapour has also been shown to amplify the greenhouse effect of other gases. Recent research suggests that atmospheric water vapour could double the warming effect of carbon dioxide.
Other trace gases, such as neon, helium, methane, krypton, hydrogen, nitrous oxide, carbon monoxide, nitrogen dioxide, ozone, xenon, sulfur dioxide and ammonia, also contribute to Earth's atmosphere. Any one of these gases makes up far less than 1% of our atmosphere. All of them have natural origins. Nitrogen, oxygen, carbon dioxide, methane are replenished through biological activity. Hydrogen, sulfur dioxide and ozone are replenished through photochemical reactions in our atmosphere. Some gases are replenished through radioactivity - argon and helium, for example. And some gases, such as xenon, neon and krypton, are expelled from deep within Earth's interior through volcanic activity.
This graphic gives you an idea of the relative amounts of gases in Earth's atmosphere:
(copyright: Cmglee (Wikipedia))
Percentages are expressed as composition by volume; ppm means parts per million.
Our atmosphere also contains traces of gases that come from industrial activity. Carbon dioxide, nitrous oxides, carbon monoxide and sulphur dioxide, in addition to natural sources, also come from man-made combustion reactions.
Carbon Monoxide Poisoning
You have probably heard of the dangers of running your car in your garage with the doors closed, or perhaps you have heard of whole families tragically killed by a faulty furnace. The killer is carbon monoxide (CO). This colourless odourless tasteless and extremely poisonous gas is very difficult to detect and so carbon monoxide detectors are recommended for every home. CO is a product of incomplete combustion, usually linked with insufficient oxygen (an enclosed space for example). In the presence of oxygen, carbon monoxide burns with a blue flame, producing carbon dioxide. This is why gas inspectors will check the flame of a gas stove when it is installed. Blue is good. Carbon monoxide binds with hemoglobin, preferentially over oxygen, so that the body cannot deliver oxygen to its cells. Air concentrations as low as 667 parts per million, will cause half of the body's hemoglobin to bind to carbon monoxide rather than oxygen. The body's cells asphyxiate, with symptoms ranging from a mild headache to death. Quick removal of CO from the air (opening the windows and doors or getting outside) can stop the deadly process and save lives. Removal to an environment with sufficient oxygen levels will allow the CO binding process to reverse itself. Follow-up treatment for severe cases is high-dose oxygen therapy in a hospital, where binding reversal can be hastened, to help avoid permanent damage, such as irreversible coma.
Ozone Pollution
It is important to distinguish stratospheric ozone (often called "good" ozone, which we will discuss shortly) from ozone in the lower atmosphere, that is, from the surface up to an altitude of about 2 kilometres. This surface ozone is created by industrial activity and it is a pollutant. It is used as a bleach, a deodorizer and a sterilizer and it is toxic. It is also a product of internal combustion engines and power plants. Nitrous oxides and volatile organic compounds from these activities react with oxygen, especially on hot sunny days, and form ozone as a result. It is a very corrosive gas that can damage the alveoli in your lungs, leading to respiratory infections and inflamed tissues, and aggravating conditions such as asthma. It can also destroy crops and forest vegetation. Some of the highest recorded ozone levels have occurred right here in Canada, in the Toronto area. Much of our ozone pollution drifts up from the U.S. industrial belt. This graphic shows afternoon ground-level ozone concentration for last July:
(units are parts per billion volume, calculated using Harvard GEOS-CHEM model, image courtesy World Meteorological Organization)
The USDA has done extensive research on ozone plant damage, showing it to be a serious and current threat to North American crops.
Various chlorofluorocarbons, carbon tetrachloride, and carbon tetrafluoride, also considered trace atmospheric gases, come strictly from industrial activities and, although they are very minute components of our atmosphere, their levels are increasing and they are potent atmospheric pollutants. Chlorofluorocarbons provide an excellent cautionary tale about the unexpected environmental damage human activities can sometimes cause.
Chlorofluorocarbons
You might remember these gases as Freons ®, the brand name given by their maker, Dupont. These gases were very useful as refrigerants, solvents and propellants and came into widespread use in the late 1900's. As early as the 1930's, these gases were used in refrigeration and as fire retardants, and atmospheric concentrations began to rise rapidly. In the 1970's, researchers discovered a disturbing link between these chemicals and the depletion of Earth's natural ozone layer high up in the atmosphere (the stratosphere). The ozone layer helps protect Earth's surface from deadly ultraviolet radiation, especially UV-C and UV-B radiation, which are most damaging to living things, as shown by this graph:
The ozone layer is replenished naturally through oxygen (O2) reactions. The chlorine ion in chlorofluorocarbons is released in the atmosphere by a photo-induced fission of the Cl-C bond. This ion catalyzes the conversion reaction of ozone into oxygen molecules. Ozone (O3) absorbs UV radiation much better than oxygen (O2) does, so this is why the growing holes in our ozone layer, centered about the two poles, is dangerous to life. The use of chlorofluorocarbons has been almost completely phased out as this writing (2012). Chlorofluorocarbons break down quite readily in the atmosphere. And if they are released lower in the atmosphere they break up without doing damage to the ozone layer. Just the same, the breakdown of stratospheric chlorofluorocarbons has led to much higher amounts of chlorine ions than originally predicted, and these ions remain stable in the atmosphere as they continue to weaken the ozone layer. Ozone depletion has been linked to increased rates of skin cancer, cataracts, damage to plants and reduction in plankton populations, particularly at latitudes over 35°.
You might have heard of other gases in the media that are being closely monitored for their potential link to global warming, such as methane, carbon dioxide and nitrous oxides. These are all greenhouse gases. They tend to absorb heat from the Sun and trap it in our atmosphere, much like greenhouse glass does. We will explore their potential threat in a future atmosphere article focusing on global warming and climate change.
Acid Rain
Rain is naturally mildly acidic. Carbon dioxide in the air reacts with water to form weak carbonic acid. The threat of acid rain you have probably heard about is an enhanced effect, a result of some of the gases emitted when fossil fuels are burned, particularly sulphur dioxide and nitrous oxides. Fossil fuels often contain sulphur impurities that burn and create sulphur dioxide. Sulphur dioxide reacts with water to create a weak solution of sulphuric acid. Usually, nitrogen and oxygen don't react with each other in the atmosphere. But at very high temperatures, in an engine for example, a small proportion of each gas does react, to create nitrogen oxides. These oxides react with water to create a weak solution of nitric acid. When fresh water pH drops below 5, most fish eggs won't hatch and adult fish begin to die off. Aquatic insects, as well, take a severe hit, reducing the biodiversity in the freshwater ecosystem, thereby weakening it. These systems are affected not only directly by acidic rain but by acidic runoff as well. In soils, some microbes cannot handle increased acidity. Their enzymes, essential for carrying out their metabolic functions, become denatured and they die. As well, toxic metals like aluminum are released from compounds and important minerals such as magnesium and calcium are leached away from soils when pH is lowered. Crops can be fertilized and limed to counteract increasing acidity but plants in the wilderness such as natural forests suffer from the lack of nutrients that results. Humans are generally not directly affected by acid rain but our buildings are. Limestone and marble, which contain lots of calcium carbonate, erode away under acidic conditions. This is why the inscriptions on old gravestones eventually become illegible. Metals, especially iron, steel, copper and bronze, also corrode faster under acidic conditions.
Why Is The Sky Blue?
You might think that the sky is blue because of the contribution of faintly blue oxygen gas, mentioned earlier. This is what liquid oxygen looks like, to give you an idea of its colour:
(Photo courtesy Dr. Warwick Hillier, Australian National University (Wikipedia))
The sky is blue, not because of oxygen but because of how sunlight is scattered by various gas molecules in Earth's atmosphere. Sunlight is made up of all the colours of the rainbow. When all these colours are combined, they make up pure white light. Prisms (or raindrops suspended in the sky) separate white sunlight into its colours.
Notice that the blue and violet light bends more and is made up of shorter wavelengths. This light has more energy than longer wavelength red light.
When sunlight reaches Earth's atmosphere, gas molecules scatter it in all directions. Higher energy (blue) sunlight tends to scatter more than lower energy (red) sunlight. This process is called Rayleigh scattering. The sky looks blue because we see many of these scattered blue rays. Rayleigh scattering gives Earth a blue halo that is even visible from outer space, as shown in this photo taken by the International Space Station. The moon as a crescent is visible through the blue haze.:
When the Sun gets low on the horizon, sunlight must pass through even more air before it reaches our eyes. Blue light rays are scattered and rescattered many times in many directions. The surface of the Earth has also reflected and scattered the sunlight. As a result, we tend to see more white light and less blue light. As the Sun sets, even more blue light is scattered while longer wave reds and yellows are less scattered, so more of these coloured rays can travel straight to your eyes. Particulate pollution, dust and water vapour all scatter light and contribute to more dramatically colourful sunsets, such as this one in South Africa:
(copyright: Geraldbrowne (Wikipedia))
Now that we are familiar with the components that make up Earth's atmosphere, let's explore its structure, next, in Earth's Atmosphere Part 3.
Labels:
Atmosphere Series
Saturday, January 28, 2012
Earth's Atmosphere Part 3 - Structure
Earth's atmosphere is divided into five layers that are based on temperature.
Air pressure and the density of each of the gases in Earth's atmosphere steadily decrease with altitude. At the Earth's surface, the air pressure is on average 1 atmosphere (atm). At 3000 m altitude, the point where most of us begin to have trouble breathing, the air pressure is about 0.7 atm. This means that we have about 70% of the oxygen to breathe as compared to sea level. At 10,000 m, standard cruising altitude for most airliners, the air pressure is about 0.3 atm. This graph shows how air pressure decreases with altitude:
0.3 atm is just enough air pressure to keep most commercial planes flying efficiently. There is not enough oxygen to keep us alive at this altitude. The average air temperature at this altitude is -50°C so oxygen would not be our only problem. Earth's atmosphere is approximately 500 km thick, but most of the gases (90%) are concentrated in the lowermost layer closest to the surface, called the troposphere. There is no exact endpoint to the atmosphere - it just gets thinner and thinner until it disappears into space.
The layers of our atmosphere are visible from outer space, as seen by the space shuttle Endeavour:
This diagram illustrates how the layers of the atmosphere are organized:
TROPOSPHERE
This starkly beautiful image, taken by the crew on the International Space Station (ISS), shows the lowest level of the atmosphere, the troposphere, to great effect as a deep orange band:
This layer, approximately 17 km deep, ends fairly abruptly at what is called the tropopause. The troposphere is named after the Greek word "tropos" meaning "turning or mixing," an apt name that describes the turbulent activity associated with all the weather that is bound within this layer. Both pressure and temperature decrease steadily with altitude within the troposphere. The composition of this layer is fairly uniform throughout, with the exception of water vapour. Its source is at the surface of the Earth and its concentration decreases very sharply with altitude. Percentage water vapour in the air declines from a maximum concentration of 100% saturation at 100°C, which is approximately 4%, to less than 1% at 0°C. At -50°C, it is virtually zero. Keep in mind that percent water vapour is not the same measurement as percent relative humidity. This graph describes the relationship between water vapour saturation and temperature:
There is almost no water vapour in the air at 10,000 m, a good thing because cruising aircraft don't have to worry about the wings continuously icing up. Under special circumstances, however, in-flight wing icing can be very hazardous. When the wings ice up, the ice can alter the wing aerodynamics and reduce their lift force. The plane is then in danger of stalling. When water is very pure it can be super-cooled. This means that even at -40°C, water can exist at liquid droplets inside clouds. It is a rare occurrence that usually happens only in the winter. If a plane flies through these clouds, the water instantly freezes on it because there is now a surface to freeze on. Most jets have pneumatic boots that inflate and break ice off the leading surfaces.
Temperatures in the troposphere decrease from a sea level average of 15°C at the equator to about -55°C and here, where the troposphere layer is thickest, up to 20 km thick, the air at the top of the troposphere can reach -75°C. At the poles, where the troposphere layer is thinner, only 7 km thick, the minimum temperature reaches -45°C. It is also indistinct in the winter when surface temperatures can be as cold as the air 7 km up. This diagram shows this variation in the height of the troposphere:
The polar jet and the subtropical jet (light green letters) are the two main jet streams in Earth's atmosphere. We will explore how they are formed a bit later on in this article. The cells (white letters) are global convective wind currents. The height of the tropopause depends on the average temperature of the entire air mass beneath it, so it is highest at the equator, where the giant Hadley cell circulates air from the warm surface high up into the atmosphere. Exceptionally tall towering thunderclouds tend to breed around the equator fueled by strong moisture-rich Hadley cell updrafts, in a band called the intertropical convergence zone. Here, commercial flights can be challenging, as jets, unable to fly over top of these thunderheads, must instead try to weave around them. The air coming back down at around 30° latitude has little moisture left, and it this is the latitude where most of Earth's deserts exist.
Why does air get colder with altitude? First of all, most of the Sun's energy is absorbed at the Earth's surface, the lowest level of the atmosphere so to speak, so heat is concentrated there. Thermodynamics explains the rest of the answer. When a parcel of air rises, it expands because the pressure it is under decreases. When it expands, it pushes on the air around it, doing work. It doesn't gain heat, however, from its environment because its thermal conductivity is very low (the thermal conductivity is much higher for soil, water etc., than for air and this is why most of the Sun's energy goes right through the atmosphere and heats the surface). Since the parcel of air does work but does not gain heat, it loses net energy and so this is why its temperature decreases. Air temperature decreases at a rate of about 6.5°C for every km in altitude, on average.
Weather
All of Earth's weather is almost entirely confined inside the troposphere (some weather does occur in the next highest layer, the stratosphere, but the mechanisms for how this occurs are not well understood). It is confined here because all weather is driven by density (which depends on temperature and moisture) differences between different pockets of air. Many of these differences are caused by differences in the incident angle of sunlight striking different areas of Earth, so that some latitudes receive more energy input than others. The strong contrast in air temperature between the poles and the equator gives rise to a powerful global wind system called the jet stream. Outside of the tropics, instabilities in the jet stream flow give rise to storms. Within the tropics, weather systems are caused by a variety of different processes, such as seasonal wind reversal in the case of Indian monsoons. Local differences in air temperature, and therefore air density, can be caused by differences in cloud cover and surfaces with different reflectivity or moisture content. These small disparities can merge to produce larger more complex systems, such as fierce thunderstorms. As warm air, carrying moisture, rises through cooler air, it cools causing the moisture within it to condense, releasing energy (in this case latent heat of fusion). This allows the rising pocket of air to cool less than the surrounding air and so it continues to rise further. Through this process, cumulous clouds form, as shown at the far left in the diagram below.
These towering vertical clouds can develop further into cumulonimbus clouds which can contain severe convection currents, resulting in lightning and downpours. Occasionally, these clouds will develop further still into supercells. Areas of organized wind rotation, called mesocyclones, form kilometres up in the sky. Descending rainfall can drag with it an area of rapidly descending air (called a rear flank down draft) that accelerates as it approaches the ground. If it drags the mesocyclone down with it, a tornado forms. The intense low pressure and high wind speeds in these funnels cause water vapour in the air to become visible and this, along with all the debris and dust they may pick up, is why they appear whitish grey like this one:
Weather is a chaotic system in which small changes can grow to have huge effects as a whole. This is what makes weather forecasting difficult.
STRATOSPHERE
The tropopause is an inversion layer. Above this boundary, temperature increases rather than decreases with altitude and very little air mixing occurs, as there is no mechanism to drive convection. Even at this altitude, rare and beautiful clouds, called nacreous clouds, can form. They are mostly visible within two hours after sunset or before dawn, when they blaze with unbelievably bright and slowly shifting iridescent colours, as shown here:
These clouds form well above tropospheric clouds, usually during the winter at northern latitudes. They are so high up that they are fully lit by sunlight while the rest of the sky is becoming dark. The stratosphere generally has no water vapour so clouds cannot form. Occasionally, however, very powerful winds in the troposphere can drive ice crystals far up into this layer. When these crystals come into contact with temperatures of at least -85°C, unusually cold even for the lower stratosphere, these brilliant clouds can form. Deep tropical convective systems can occasionally break through the tropopause as well.
The stratosphere layer lies between the troposphere and the next layer up, the mesosphere. In this photograph, the space shuttle Endeavour straddles the stratosphere and the mesosphere:
The orange troposphere gives way to the white stratosphere and then the bluish haze of the mesosphere.
This layer of atmosphere is stratified into layers of different temperatures. Coldest layers are closest to Earth and warmer layers are farther up. The stratosphere ranges from temperatures typically around -60°C at its lowest altitude to about -3°C near the top. It is usually situated between about 10 km and 50 km altitude. Commercial jets can and often do straddle the lower stratosphere where the low temperatures increase fuel efficiency and low air density decreases drag. Almost all severe weather is safely beneath the aircraft and generally there is no turbulence to deal with. The challenge of stratospheric flight is that air here is of such low density that it does not provide much lift on the wings. As well, jet engines lose power at higher altitudes because there is less air intake into the engines. Finally, commercial jet fuels are only safe above their freezing points. On most flights, pilots descend into warmer air when their fuel temperature warning light goes off. However, on some transpolar flights, it makes more sense to ascend into warmer air. Most operators in Canada use jet fuels with a minimum temperature of -40°C, while some jet fuels used in northern Russia can be used down to -50°C. The new Boeing 787, sporting many technical innovations, will eventually be certified to fly at -55°C. There are many new designs for military stratospheric aircraft and these jets can travel at much higher altitudes than commercial airliners, most of which are light and sport huge wingspans, such as many of those in the U.S. Pentagon's Vulture Project. One prototype, an unmanned aircraft, would have a massive 150 m wingspan, fly at 27 km altitude, and stay up in the air for five years at a time, performing surveillance and communications activities. It faces many technical challenges, such as long-term exposure to extreme solar radiation, the need to minimize weight and the problem of how to power the aircraft. Many designs, this one included, make us of ample solar power at these extreme altitudes and have solar panels installed on the tops of their wings, while others are exploring the use of hydrogen fuel cells.
Stratospheric Ozone
This is the layer that contains the ozone layer, shown here as an azure blue band:
The stratification of temperature in the stratosphere comes from the absorption of the Sun's energy by ozone gas. In the upper layers, ozone (O3) absorbs UVB and UVC rays (two types of ultraviolet radiation) and splits into molecular (O2) and atomic (O) oxygen gas. This takes place through a reaction called photodissociation. Energy (in the form of UV radiation) is absorbed when ozone bonds are broken to create oxygen ions. Oxygen ions are very reactive and quickly combine with other atoms in the stratosphere. Although these reactions involve both the breaking and making of chemical bonds, they are overall exothermic, which means they release energy. That is why the upper layer, where the majority of these reactions take place, is warmest. The mid layers have less UV radiation passing through them, and less energy is released because fewer of these reactions are taking place. Some heat is released, however, because O2 and O are able to recombine here, an exothermic reaction, and this where most ozone is produced. The lowest layers are coldest because much less photochemical activity takes place there. There is no evidence yet of ozone depletion affecting stratospheric temperatures.
These stratified layers of gases are quite stable because no convective activity occurs here. Horizontal stratospheric circulation does occur however, transporting ozone and other gases. Almost all air enters the stratosphere over the tropics, and most of it moves fairly rapidly east to west around the equator and west to east towards the poles, as this 2-minute simulation shows:
Using these stratospheric conveyor belts, particles such as volcanic dust, may cover the globe in as little as two days. Volcanic ash tends to stay in the troposphere no more than a couple of weeks. Very fine volcanic tephra particles may cover the globe and remain in the stratosphere for a few months and they have only minor effects on the climate. These are generally the particles that contribute to spectacular sunsets associated with volcanic eruptions. The major climate influence from volcanic eruptions comes from gaseous sulphur compounds spewed into the atmosphere, especially sulphur dioxide, which reacts with water in the air to create sulphate aerosols. These very fine particles can stay suspended in the stratosphere for 2-3 years, where they can produce a strong climate cooling effect by reflecting sunlight and modifying clouds as the particles eventually sift down through the atmosphere, contributing to increased cloud cover. This graphic describes the atmospheric effects a typical volcanic eruption:
Looking at this graphic, you may notice that volcanic eruptions are a natural cause of ozone depletion. The ozone layer has naturally replenished itself over the eons through the reactions described above, which tend to maintain an equilibrium concentration of ozone. Chlorofluorocarbons, as well as some other pollutants, overwhelm this natural equilibrium and that is why ozone is being depleted from the stratosphere. Chlorofluorocarbons are discussed in the previous atmosphere article on the composition of the atmosphere. No one is entirely sure how old the ozone layer is, but four billion years ago there was no oxygen, and no ozone, in Earth's atmosphere. When photosynthetic algae began to colonize the oceans, they pumped out oxygen and eventually it rose in the atmosphere. Ozone was created as extreme UV radiation broke oxygen down. Without the ozone layer, deadly UV radiation, especially UVC, would have prevented life from colonizing land on Earth.
Stratospheric Radioactive Fallout
Thousands of atmospheric nuclear tests have been carried out all over the world and there have been extensive studies of both local low level atmospheric radioactive particulates and radioactive dust that travels on the jet streams in the troposphere. This fallout is eventually washed to the surface in rain. Stratospheric radioactive dust, however, can remain airborne for years, and is an even greater concern. As I understand them these tests are not easy to carry out as there are so many variables involved in predicting where this dust may end up. It is much more difficult than predicting long term weather patterns. If you are interested in finding out what we do know about stratospheric radioactive fallout, I recommend this scientific paper released by the U.S. Air Force Institute of Technology in 1987. If you would like to know more about radiation and what it is, I recommend my article, "Radiation: What is Happening In Japan."
Stratospheric Life
Perhaps surprisingly, bacteria survive in the stratosphere, making it part of Earth's biosphere. In 2009, a group of Indian scientists discovered three species of upper stratospheric bacteria not found on Earth's surface and highly resistant to UV radiation. They were collected from a special balloon that took samples of air from different altitudes ranging from 20 km to 41 km. Of course, the scientists had to be meticulous to avoid any possible contamination of terrestrial species, and the 2009 test was a carefully executed repeat of a 2001 experiment, which also found the bacteria.
Occasionally, some species of migrating birds can straddle this zone. Birds have extremely efficient lungs that can extract much more oxygen from the air than our lungs can, giving them a great advantage in high altitude flight. Although most birds tend to fly below 150 metres, many migrating birds, for example, Tundra swans and Whooping swans have been observed flying alongside jetliners cruising at around 7500 m. Bar-headed geese have been observed migrating over the top of Mount Everest, the peak of which stands at almost 9000 m. For humans this is well within what is called the death zone, 8000 m and up. There is only a third of oxygen in the air compared to seal level, and humans cannot acclimatize to this level. Extreme climbers need supplemental tanks of O2.
MESOSPHERE
Unlike the stratosphere, temperatures in the mesosphere decrease with altitude. Temperatures at the upper boundary of the mesosphere, the mesopause, are the coldest temperatures associated with Earth, around -145°C. This layer extends from around 50 km to 100 km in altitude, and like the layers below it, its location is affected by the seasons and by latitude. Temperatures decrease with altitude in this layer because heating by UV absorption of ozone falls off and, more significantly, increasing cooling through carbon dioxide radiative emission occurs. This is how it works: carbon dioxide, like the other greenhouse gases - water vapour, methane and nitrous oxide - is made up of two or more atoms bound loosely enough together to be able to vibrate when they absorb radiation (energy) N2 and O2 gases are too tightly bound to absorb energy this way so they are not greenhouse gases. Eventually these greenhouse molecules emit the radiation again. This absorption-emission-absorption cycle keeps heat near Earth's surface, by reradiating heat in all directions and reducing the heat radiated back out into space. The mesosphere is almost a vacuum, and with so few molecules around to absorb CO2's emissions, there is a net loss of energy as CO2 radiates energy into space.
Clouds, Sprites, Jets and Meteors
And, yet even here in the almost-vacuum of the mesosphere, special clouds, called noctilucent clouds, can form. Their formation requires a temperature of -90°C or lower at an altitude of around 85 km. Like stratospheric nacreous clouds, these clouds are best observed at high latitudes, between 50 and 65°. But unlike nacreous clouds, these are more likely to develop on summer nights. They tend to be bluish-white and rich with undulations. In this photograph, these bright, sharp and eerie clouds glow well after sunset:
They are made of tiny crystals of water ice and dust. The dust might come from micrometeors or from volcanic eruptions. The water could be formed from the reaction of methane with hydroxyl radicals in the stratosphere, or even the exhaust from the space shuttles. These clouds are rare because water is so rare at this altitude, and what little there is tends to be broken down by UV radiation from the Sun. The mesosphere contains about 1 hundred millionth that of the air moisture in the Sahara desert. These clouds were first known to be observed in 1885, two years after Krakatoa erupted. It is not known if this was a coincidence or not, but more and more of these clouds have been observed since then. Their relatively recent appearance may be linked to climate change, but again, the link is not clear. It is difficult to study this layer of the atmosphere because it is above the maximum altitude for almost all aircraft and below the minimum altitude for orbital spacecraft.
Other mysterious phenomena, such as red sprites and blue jets, occur in the mesosphere as well.
Red sprites, which look like bright reddish-orange flashes, are large-scale electrical discharges that occur high above thunderstorm clouds. This is the first colour image captured of one by NASA aircraft in 1994:
They are often associated with bluish white tendrils hanging below and arcing branches above. Despite often being categorized as such, sprites are not lightning. They are cold plasma phenomena, a bit like a fluorescent tube discharge, that are triggered by lightning in the troposphere below. The physical mechanism responsible for sprite production is still unknown but they seem to be linked to Earth's electrical field system and they may be part of every medium to large thunderstorm.
Blue Jets
Whereas sprites tend to form well above the tops of thunderstorm clouds, blue jets tend to project directly upward from them, usually as a narrow cone. These phenomena tend to form, as a result, lower in the atmosphere, often straddling the stratosphere/mesosphere boundary, as shown in this image which compares them with red sprites and lightning:
While sprites seem to be triggered by lightning strikes, blue jets appear to be more strongly associated with intense hail activity. Blue jets are believed to be the result of blue emission lines from neutral or ionized molecular (N2) nitrogen gas. Blue jets are much rarer than red sprites.
Millions of meteors enter Earth's atmosphere every day, with most of them melting or vapourizing altogether as they collide with gas molecules and atoms in the mesosphere. The vast majority of meteors are the size of a pebble or smaller, and their glow as they disintegrate is not even visible from the ground. All these meteor collisions add up to about 50 metric tons of material striking the atmosphere every day, almost all of which evaporates, leaving sparse metal layers such as sodium and potassium in the mesosphere as well as iron oxides and silica-rich nano size particles. These particles, studied using radar data and rocket-borne in situ techniques, are believed to be at extremely low densities in the mesosphere but they may be what nucleates the rare noctilucent clouds that form in this layer.
THERMOSPHERE
The thermosphere is the thickest of all the atmospheric layers, beginning between 80 and 100 km above Earth and extending to between 500 and 1000 km. Its thickness depends on solar activity. For example, it experienced an unusually severe collapse during a recent deep solar minimum in 2008-2009. This layer of atmosphere, the realm of meteors, auroras and satellites, is where solar radiation makes its first contact with Earth. When solar activity is high, UV radiation from the Sun warms the thermosphere, causing it to "puff up like a marshmallow held over a campfire." The opposite happens during low solar activity. The recent contraction, now recovering, was far more intense than the ebb in solar activity can explain, suggesting that we don't yet fully understand all the dynamics of this layer.
Named after the Greek word for heat, the thermosphere can reach temperatures as high as 1500°C during the daytime and when solar activity is at a maximum. A thermometer would not be able to record this heat, however, because the energy lost through thermal radiation would overwhelm the energy transferred from the atmospheric gas. In fact, atoms and molecules are so few and far between in this layer, that there is little to no heat transfer possible between them. This means that even though individual atoms are highly energized in the sunlight, a sensory surface could not "feel" that as heat.
Below the thermosphere, all the atmospheric gases mentioned in the preceding article are mixed together by turbulence, even in the stratosphere and mesosphere where some stratification becomes evident. In this layer, however, different gases tend to form separate layers (with little or no interaction between them) based on their atomic weights. This layer, as a whole, contains mostly molecular oxygen, molecular nitrogen, atomic oxygen, atomic nitrogen and helium gases. At the lowest level, molecular nitrogen and oxygen gases exist in much the usual percentages. Molecules of oxygen and nitrogen are relatively heavier than the other gases, however, and their atmospheric levels tend to fall off as altitude increases. Lighter atomic oxygen and nitrogen gases, and then gases such as hydrogen and helium, become relatively more abundant in the uppermost reaches of the thermosphere. This graphic explains what happens in the thermosphere:
As you can see, much of the most energetic, and therefore deadly, radiation from the Sun, such as extreme and far UV radiation, as well as some X-ray radiation, is absorbed and blocked out by the thermosphere. As mentioned, when solar radiation is high, this layer heats up and expands. When it expands it becomes less dense. Although this layer contains on average an extremely low density of gases, changes in density must be taken into account when engineers calculate the orbits of satellites. Higher density contributes to increased drag on these fast-moving bodies and those effects must be offset occasionally by brief boosts from their onboard rockets to keep their orbits from decaying and spiraling down to Earth. The International Space Station (ISS) orbits in the thermosphere at between 320 and 380 km altitude. Here it is, docked with the space shuttle Endeavour, in this May 2011 photograph:
This was the last space shuttle docking with the ISS, and the last mission for the Endeavour.
Although the gases in this layer are stratified, they do circulate thanks to diurnal heating and cooling, creating waves and tides, not unlike ocean tides. Gas ions as well as free electrons and protons, all products of the splitting of gas molecules and atoms by extreme radiation, move along in these tides and collide with neutral gases to produce powerful electrical currents in some parts of the thermosphere. In addition to these currents, and interacting with them, are the aurorae. Aurorae (the Northern and Southern Lights) occur when solar wind, itself made up of highly energized electrons, protons and other ions, collide with ions, atoms and molecules in the thermosphere at high latitudes. The atmospheric atoms and molecules become highly energized as a result and they shed this energy by emitting light at specific wavelengths, depending on the atom or molecule that's energized. For example, excited oxygen molecules emit bright red light and nitrogen molecules emit reddish purple light. The most common colour for aurorae is green and that comes from excited oxygen atoms, as in these Southern Lights captured in 1994:
If you would like to more about how the aurorae work, please see my article called "The Northern Lights."
EXOSPHERE
This is the uppermost layer of Earth's atmosphere, beginning at around 500 km altitude. Its lower boundary with the thermosphere, called the exobase or critical level, is highly variable, depending on how expanded the thermosphere is beneath it. Here there are basically no atomic collisions, because atoms are so far apart from each other. Collisions dominate the motion of gas atoms and molecules beneath this boundary, but above it atoms are governed by ballistic motion, and with sufficient velocity they can and do escape Earth's gravity. It all depends on the velocity and trajectory. Other atoms, with the right trajectory and velocity, orbit Earth a long time, as satellite gases.
The upper boundary of the exosphere extends half the way to the Moon, about 190,000 km. It is defined as the distance where the influence of solar radiation on average atomic hydrogen velocity overcomes the gravitational pull by the Earth. In fact, the exosphere consists almost entirely of neutral hydrogen atoms, the lightest of all the atmospheric gases. Earth's magnetic field protects our atmosphere from being stripped away by solar wind. It acts like an energy collector that interacts with the solar wind material and draws energy out of it. However, Earth's magnetic field also funnels that energy and guides it into the upper atmosphere (this occurs at the poles) and allowing atoms and molecules to escape through the same funnels. There is no cause for alarm, though, because the rate of atmospheric loss through both gravitational escape and escape through the magnetic field is so low, it would take until the Sun becomes a red giant, billions of years from now, to lose appreciable atmosphere.
The exosphere is UV-visible from outer space as a geocorona, which extends to about 100,000 km. This is solar far-ultraviolet light that reflects off of neutral hydrogen atoms in the atmosphere. You cannot see it with the naked eye - Earth's atmosphere appears to end sharply as seen against black outer space:
However, the atmospheric edge looks completely different when seen using UV light instead of visible light. Below is a colour enhancement of an ultraviolet photograph of Earth, with its geocorona extending far out in all directions. Sunlight is shining from the left and the geocorona is brighter on that side. You can make out Earth as outlined by the curvature of the yellow area:
The exosphere plays an important role in the plasma budget of Earth's magnetosphere. It acts as a sink for charged particles, and there is a great influx of them during geomagnetic storms. These charged particles can exchange energy with exospheric neutral hydrogen, allowing them to return to their ground states, removing plasma and restoring the exosphere to its pre-storm state.
IONOSPHERE
Another layer called the ionosphere overlaps part of the mesosphere, thermosphere and exosphere, as shown here:
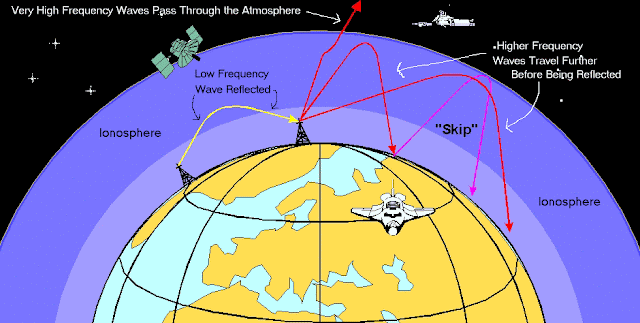
"F" and "E" refer to ionospheric layers. The lowest D layer is not shown. Net ionization in the D layer is quite low in the daytime and disappears altogether at night. However, there are enough free electrons present to collide with high frequency radio waves and absorb some of their energy during the daytime. This is why you can sometimes pick up very distant AM radio stations at night but not in the daytime. The E layer is the middle layer of the ionosphere and it tends to be more reflective than absorptive. After sunset, the height of the top of this layer increases so that radio waves can travel extra far, by reflecting off of it. Ham radio operators (like my dad) get especially excited when small thin clouds of extra-intense ionization are sometimes set up, usually during the summer, and radio propagation paths that are usually unreachable open up, allowing for skip distances of up 1000 km, single hop distances of between 1000 and 2500 km and very rare double hop distances of over 3500 km. This reflection is just like a rock skipping off water. The F layer above is located in the thermosphere and has the highest concentration of free electrons and ions anywhere in the atmosphere. This layer is a dependable reflector of radio waves that is not affected by atmospheric conditions unlike the E layer below, allowing for skywave propagation. Amateur hams back in the1920's, with limited transmitter power, relied on this layer for long distance communication. This graphic shows in general how HF radio waves travel through the atmosphere:
VLF (very low frequency) radio waves can be used to monitor sudden ionic disturbances in the atmosphere. In the daytime, these longer wavelengths dependably bounce off the D layer (image below right). Here, unusual reception patterns can be used to observe the way the ionosphere has been affected by X-ray flares from the Sun. In this way, Sun activity can be monitored. At might, when the D layer disappears, they are reflected by higher E and F layers. When this occurs, VLF wave propagation is strongly affected by ionospheric disturbances, leading to signal variations that are large enough to make monitoring sudden ionic disturbances impossible. This graphic compares daytime and nighttime VLF propagation modes:
The structure of the ionosphere is distributed by gravity waves so the reflective surfaces of ionization can be wavy. This is why ham signals tend to fade in and out and sound fluttery. Gravity waves are an interesting phenomenon. Any fluid, such as water or air, can generate gravity waves when a trigger like an updraft causes a pocket of air to be displaced vertically in stable air. Because of momentum it will overshoot its rise, and then overshoot its subsequent sinking. This motion sets up a series of waves as the air tries to regain equilibrium, as shown here:
This phenomenon also happens with the charged ions of the ionosphere, which also act like a fluid.
During an intense solar flare, hard X-rays from the Sun can reach down into the D layer, greatly increase radio wave absorption and cause radio blackout. Geomagnetic storms can fragment and even temporarily destroy much of the F layer altogether. When this happens, radio transmission becomes sporadic and unpredictable. Ham, ground to air and ship to shore communications are usually affected. During intense geomagnetic storms, communications satellites can be damaged by the influx of plasma higher up in the thermosphere causing disruptions to telephone, TV, GPS and Internet service.
This is the layer of atmosphere that is ionized by solar radiation and forms the inner edge of the magnetosphere. It is a shell of electrons, charged atoms and charged molecules, a ring of plasma in other words, that stretches from about 50 km altitude all the way to the edge of the exosphere, 1000 km. These particles are charged, or ionized, by (mostly) UV radiation from the Sun. In the thermosphere, free electrons can exist for some time before they are captured by a positive ion. It is these electrons that affect radio propagation. And it is this plasma ring that becomes energized during geomagnetic storms. UV, and other kinds of sufficiently energetic radiation can dislodge an electron from a gas atom or molecule, ionizing it. The electron that is released has a great deal of energy and this is the energy that contributes to the high temperatures found in the thermosphere, for example. Free electrons are eventually captured by positive ions, a reverse of the ionization process. At lower levels in the atmosphere, this recombination process prevails because gas molecules, atoms and ions are much closer together. The outer layers tend to remain ionized. The ring current, and Earth's magnetosphere as a whole, forms a complex relationship between Earth's atmosphere and solar activity. This diagram gives you an idea of where the ring current is situated with respect to Earth:
This animation shows how Earth's magnetic field interacts with the solar wind (click on it):
The outermost white lines (magnetic field lines) in this image correspond to the outer capsule (the magnetopause) in the image above this one.
Increased solar wind from a solar storm temporarily compresses the magnetosphere. At this point the thermosphere puffs up and becomes less dense. Eventually the tailward magnetic field lines (all shown as white lines extending outward on the night side of Earth) collapse and Earth's magnetosphere recovers, with the help of the ionization sink of the exosphere.
Now that we are familiar with Earth's atmosphere, we can compare its dynamics with those of other planets, next, in Earth's Atmosphere Part 4.
Air pressure and the density of each of the gases in Earth's atmosphere steadily decrease with altitude. At the Earth's surface, the air pressure is on average 1 atmosphere (atm). At 3000 m altitude, the point where most of us begin to have trouble breathing, the air pressure is about 0.7 atm. This means that we have about 70% of the oxygen to breathe as compared to sea level. At 10,000 m, standard cruising altitude for most airliners, the air pressure is about 0.3 atm. This graph shows how air pressure decreases with altitude:
(created by Geek.not.nerd (Wikipedia))
0.3 atm is just enough air pressure to keep most commercial planes flying efficiently. There is not enough oxygen to keep us alive at this altitude. The average air temperature at this altitude is -50°C so oxygen would not be our only problem. Earth's atmosphere is approximately 500 km thick, but most of the gases (90%) are concentrated in the lowermost layer closest to the surface, called the troposphere. There is no exact endpoint to the atmosphere - it just gets thinner and thinner until it disappears into space.
The layers of our atmosphere are visible from outer space, as seen by the space shuttle Endeavour:
This diagram illustrates how the layers of the atmosphere are organized:
(copyright: The Ozone Hole website)
TROPOSPHERE
This starkly beautiful image, taken by the crew on the International Space Station (ISS), shows the lowest level of the atmosphere, the troposphere, to great effect as a deep orange band:
This layer, approximately 17 km deep, ends fairly abruptly at what is called the tropopause. The troposphere is named after the Greek word "tropos" meaning "turning or mixing," an apt name that describes the turbulent activity associated with all the weather that is bound within this layer. Both pressure and temperature decrease steadily with altitude within the troposphere. The composition of this layer is fairly uniform throughout, with the exception of water vapour. Its source is at the surface of the Earth and its concentration decreases very sharply with altitude. Percentage water vapour in the air declines from a maximum concentration of 100% saturation at 100°C, which is approximately 4%, to less than 1% at 0°C. At -50°C, it is virtually zero. Keep in mind that percent water vapour is not the same measurement as percent relative humidity. This graph describes the relationship between water vapour saturation and temperature:
(copyright: GregBenson (en.wikipedia))
There is almost no water vapour in the air at 10,000 m, a good thing because cruising aircraft don't have to worry about the wings continuously icing up. Under special circumstances, however, in-flight wing icing can be very hazardous. When the wings ice up, the ice can alter the wing aerodynamics and reduce their lift force. The plane is then in danger of stalling. When water is very pure it can be super-cooled. This means that even at -40°C, water can exist at liquid droplets inside clouds. It is a rare occurrence that usually happens only in the winter. If a plane flies through these clouds, the water instantly freezes on it because there is now a surface to freeze on. Most jets have pneumatic boots that inflate and break ice off the leading surfaces.
Temperatures in the troposphere decrease from a sea level average of 15°C at the equator to about -55°C and here, where the troposphere layer is thickest, up to 20 km thick, the air at the top of the troposphere can reach -75°C. At the poles, where the troposphere layer is thinner, only 7 km thick, the minimum temperature reaches -45°C. It is also indistinct in the winter when surface temperatures can be as cold as the air 7 km up. This diagram shows this variation in the height of the troposphere:
The polar jet and the subtropical jet (light green letters) are the two main jet streams in Earth's atmosphere. We will explore how they are formed a bit later on in this article. The cells (white letters) are global convective wind currents. The height of the tropopause depends on the average temperature of the entire air mass beneath it, so it is highest at the equator, where the giant Hadley cell circulates air from the warm surface high up into the atmosphere. Exceptionally tall towering thunderclouds tend to breed around the equator fueled by strong moisture-rich Hadley cell updrafts, in a band called the intertropical convergence zone. Here, commercial flights can be challenging, as jets, unable to fly over top of these thunderheads, must instead try to weave around them. The air coming back down at around 30° latitude has little moisture left, and it this is the latitude where most of Earth's deserts exist.
Why does air get colder with altitude? First of all, most of the Sun's energy is absorbed at the Earth's surface, the lowest level of the atmosphere so to speak, so heat is concentrated there. Thermodynamics explains the rest of the answer. When a parcel of air rises, it expands because the pressure it is under decreases. When it expands, it pushes on the air around it, doing work. It doesn't gain heat, however, from its environment because its thermal conductivity is very low (the thermal conductivity is much higher for soil, water etc., than for air and this is why most of the Sun's energy goes right through the atmosphere and heats the surface). Since the parcel of air does work but does not gain heat, it loses net energy and so this is why its temperature decreases. Air temperature decreases at a rate of about 6.5°C for every km in altitude, on average.
Weather
All of Earth's weather is almost entirely confined inside the troposphere (some weather does occur in the next highest layer, the stratosphere, but the mechanisms for how this occurs are not well understood). It is confined here because all weather is driven by density (which depends on temperature and moisture) differences between different pockets of air. Many of these differences are caused by differences in the incident angle of sunlight striking different areas of Earth, so that some latitudes receive more energy input than others. The strong contrast in air temperature between the poles and the equator gives rise to a powerful global wind system called the jet stream. Outside of the tropics, instabilities in the jet stream flow give rise to storms. Within the tropics, weather systems are caused by a variety of different processes, such as seasonal wind reversal in the case of Indian monsoons. Local differences in air temperature, and therefore air density, can be caused by differences in cloud cover and surfaces with different reflectivity or moisture content. These small disparities can merge to produce larger more complex systems, such as fierce thunderstorms. As warm air, carrying moisture, rises through cooler air, it cools causing the moisture within it to condense, releasing energy (in this case latent heat of fusion). This allows the rising pocket of air to cool less than the surrounding air and so it continues to rise further. Through this process, cumulous clouds form, as shown at the far left in the diagram below.
These towering vertical clouds can develop further into cumulonimbus clouds which can contain severe convection currents, resulting in lightning and downpours. Occasionally, these clouds will develop further still into supercells. Areas of organized wind rotation, called mesocyclones, form kilometres up in the sky. Descending rainfall can drag with it an area of rapidly descending air (called a rear flank down draft) that accelerates as it approaches the ground. If it drags the mesocyclone down with it, a tornado forms. The intense low pressure and high wind speeds in these funnels cause water vapour in the air to become visible and this, along with all the debris and dust they may pick up, is why they appear whitish grey like this one:
Weather is a chaotic system in which small changes can grow to have huge effects as a whole. This is what makes weather forecasting difficult.
STRATOSPHERE
The tropopause is an inversion layer. Above this boundary, temperature increases rather than decreases with altitude and very little air mixing occurs, as there is no mechanism to drive convection. Even at this altitude, rare and beautiful clouds, called nacreous clouds, can form. They are mostly visible within two hours after sunset or before dawn, when they blaze with unbelievably bright and slowly shifting iridescent colours, as shown here:
These clouds form well above tropospheric clouds, usually during the winter at northern latitudes. They are so high up that they are fully lit by sunlight while the rest of the sky is becoming dark. The stratosphere generally has no water vapour so clouds cannot form. Occasionally, however, very powerful winds in the troposphere can drive ice crystals far up into this layer. When these crystals come into contact with temperatures of at least -85°C, unusually cold even for the lower stratosphere, these brilliant clouds can form. Deep tropical convective systems can occasionally break through the tropopause as well.
The stratosphere layer lies between the troposphere and the next layer up, the mesosphere. In this photograph, the space shuttle Endeavour straddles the stratosphere and the mesosphere:
The orange troposphere gives way to the white stratosphere and then the bluish haze of the mesosphere.
This layer of atmosphere is stratified into layers of different temperatures. Coldest layers are closest to Earth and warmer layers are farther up. The stratosphere ranges from temperatures typically around -60°C at its lowest altitude to about -3°C near the top. It is usually situated between about 10 km and 50 km altitude. Commercial jets can and often do straddle the lower stratosphere where the low temperatures increase fuel efficiency and low air density decreases drag. Almost all severe weather is safely beneath the aircraft and generally there is no turbulence to deal with. The challenge of stratospheric flight is that air here is of such low density that it does not provide much lift on the wings. As well, jet engines lose power at higher altitudes because there is less air intake into the engines. Finally, commercial jet fuels are only safe above their freezing points. On most flights, pilots descend into warmer air when their fuel temperature warning light goes off. However, on some transpolar flights, it makes more sense to ascend into warmer air. Most operators in Canada use jet fuels with a minimum temperature of -40°C, while some jet fuels used in northern Russia can be used down to -50°C. The new Boeing 787, sporting many technical innovations, will eventually be certified to fly at -55°C. There are many new designs for military stratospheric aircraft and these jets can travel at much higher altitudes than commercial airliners, most of which are light and sport huge wingspans, such as many of those in the U.S. Pentagon's Vulture Project. One prototype, an unmanned aircraft, would have a massive 150 m wingspan, fly at 27 km altitude, and stay up in the air for five years at a time, performing surveillance and communications activities. It faces many technical challenges, such as long-term exposure to extreme solar radiation, the need to minimize weight and the problem of how to power the aircraft. Many designs, this one included, make us of ample solar power at these extreme altitudes and have solar panels installed on the tops of their wings, while others are exploring the use of hydrogen fuel cells.
Stratospheric Ozone
This is the layer that contains the ozone layer, shown here as an azure blue band:
(Image is from netonnet.wordpress, in an excellent article outlining what we can do as individuals to protect the ozone layer)
The stratification of temperature in the stratosphere comes from the absorption of the Sun's energy by ozone gas. In the upper layers, ozone (O3) absorbs UVB and UVC rays (two types of ultraviolet radiation) and splits into molecular (O2) and atomic (O) oxygen gas. This takes place through a reaction called photodissociation. Energy (in the form of UV radiation) is absorbed when ozone bonds are broken to create oxygen ions. Oxygen ions are very reactive and quickly combine with other atoms in the stratosphere. Although these reactions involve both the breaking and making of chemical bonds, they are overall exothermic, which means they release energy. That is why the upper layer, where the majority of these reactions take place, is warmest. The mid layers have less UV radiation passing through them, and less energy is released because fewer of these reactions are taking place. Some heat is released, however, because O2 and O are able to recombine here, an exothermic reaction, and this where most ozone is produced. The lowest layers are coldest because much less photochemical activity takes place there. There is no evidence yet of ozone depletion affecting stratospheric temperatures.
These stratified layers of gases are quite stable because no convective activity occurs here. Horizontal stratospheric circulation does occur however, transporting ozone and other gases. Almost all air enters the stratosphere over the tropics, and most of it moves fairly rapidly east to west around the equator and west to east towards the poles, as this 2-minute simulation shows:
Using these stratospheric conveyor belts, particles such as volcanic dust, may cover the globe in as little as two days. Volcanic ash tends to stay in the troposphere no more than a couple of weeks. Very fine volcanic tephra particles may cover the globe and remain in the stratosphere for a few months and they have only minor effects on the climate. These are generally the particles that contribute to spectacular sunsets associated with volcanic eruptions. The major climate influence from volcanic eruptions comes from gaseous sulphur compounds spewed into the atmosphere, especially sulphur dioxide, which reacts with water in the air to create sulphate aerosols. These very fine particles can stay suspended in the stratosphere for 2-3 years, where they can produce a strong climate cooling effect by reflecting sunlight and modifying clouds as the particles eventually sift down through the atmosphere, contributing to increased cloud cover. This graphic describes the atmospheric effects a typical volcanic eruption:
Looking at this graphic, you may notice that volcanic eruptions are a natural cause of ozone depletion. The ozone layer has naturally replenished itself over the eons through the reactions described above, which tend to maintain an equilibrium concentration of ozone. Chlorofluorocarbons, as well as some other pollutants, overwhelm this natural equilibrium and that is why ozone is being depleted from the stratosphere. Chlorofluorocarbons are discussed in the previous atmosphere article on the composition of the atmosphere. No one is entirely sure how old the ozone layer is, but four billion years ago there was no oxygen, and no ozone, in Earth's atmosphere. When photosynthetic algae began to colonize the oceans, they pumped out oxygen and eventually it rose in the atmosphere. Ozone was created as extreme UV radiation broke oxygen down. Without the ozone layer, deadly UV radiation, especially UVC, would have prevented life from colonizing land on Earth.
Stratospheric Radioactive Fallout
Thousands of atmospheric nuclear tests have been carried out all over the world and there have been extensive studies of both local low level atmospheric radioactive particulates and radioactive dust that travels on the jet streams in the troposphere. This fallout is eventually washed to the surface in rain. Stratospheric radioactive dust, however, can remain airborne for years, and is an even greater concern. As I understand them these tests are not easy to carry out as there are so many variables involved in predicting where this dust may end up. It is much more difficult than predicting long term weather patterns. If you are interested in finding out what we do know about stratospheric radioactive fallout, I recommend this scientific paper released by the U.S. Air Force Institute of Technology in 1987. If you would like to know more about radiation and what it is, I recommend my article, "Radiation: What is Happening In Japan."
Stratospheric Life
Perhaps surprisingly, bacteria survive in the stratosphere, making it part of Earth's biosphere. In 2009, a group of Indian scientists discovered three species of upper stratospheric bacteria not found on Earth's surface and highly resistant to UV radiation. They were collected from a special balloon that took samples of air from different altitudes ranging from 20 km to 41 km. Of course, the scientists had to be meticulous to avoid any possible contamination of terrestrial species, and the 2009 test was a carefully executed repeat of a 2001 experiment, which also found the bacteria.
Occasionally, some species of migrating birds can straddle this zone. Birds have extremely efficient lungs that can extract much more oxygen from the air than our lungs can, giving them a great advantage in high altitude flight. Although most birds tend to fly below 150 metres, many migrating birds, for example, Tundra swans and Whooping swans have been observed flying alongside jetliners cruising at around 7500 m. Bar-headed geese have been observed migrating over the top of Mount Everest, the peak of which stands at almost 9000 m. For humans this is well within what is called the death zone, 8000 m and up. There is only a third of oxygen in the air compared to seal level, and humans cannot acclimatize to this level. Extreme climbers need supplemental tanks of O2.
MESOSPHERE
Unlike the stratosphere, temperatures in the mesosphere decrease with altitude. Temperatures at the upper boundary of the mesosphere, the mesopause, are the coldest temperatures associated with Earth, around -145°C. This layer extends from around 50 km to 100 km in altitude, and like the layers below it, its location is affected by the seasons and by latitude. Temperatures decrease with altitude in this layer because heating by UV absorption of ozone falls off and, more significantly, increasing cooling through carbon dioxide radiative emission occurs. This is how it works: carbon dioxide, like the other greenhouse gases - water vapour, methane and nitrous oxide - is made up of two or more atoms bound loosely enough together to be able to vibrate when they absorb radiation (energy) N2 and O2 gases are too tightly bound to absorb energy this way so they are not greenhouse gases. Eventually these greenhouse molecules emit the radiation again. This absorption-emission-absorption cycle keeps heat near Earth's surface, by reradiating heat in all directions and reducing the heat radiated back out into space. The mesosphere is almost a vacuum, and with so few molecules around to absorb CO2's emissions, there is a net loss of energy as CO2 radiates energy into space.
Clouds, Sprites, Jets and Meteors
And, yet even here in the almost-vacuum of the mesosphere, special clouds, called noctilucent clouds, can form. Their formation requires a temperature of -90°C or lower at an altitude of around 85 km. Like stratospheric nacreous clouds, these clouds are best observed at high latitudes, between 50 and 65°. But unlike nacreous clouds, these are more likely to develop on summer nights. They tend to be bluish-white and rich with undulations. In this photograph, these bright, sharp and eerie clouds glow well after sunset:
(copyright: Martin Koitmae (Wikipedia))
They are made of tiny crystals of water ice and dust. The dust might come from micrometeors or from volcanic eruptions. The water could be formed from the reaction of methane with hydroxyl radicals in the stratosphere, or even the exhaust from the space shuttles. These clouds are rare because water is so rare at this altitude, and what little there is tends to be broken down by UV radiation from the Sun. The mesosphere contains about 1 hundred millionth that of the air moisture in the Sahara desert. These clouds were first known to be observed in 1885, two years after Krakatoa erupted. It is not known if this was a coincidence or not, but more and more of these clouds have been observed since then. Their relatively recent appearance may be linked to climate change, but again, the link is not clear. It is difficult to study this layer of the atmosphere because it is above the maximum altitude for almost all aircraft and below the minimum altitude for orbital spacecraft.
Other mysterious phenomena, such as red sprites and blue jets, occur in the mesosphere as well.
Red sprites, which look like bright reddish-orange flashes, are large-scale electrical discharges that occur high above thunderstorm clouds. This is the first colour image captured of one by NASA aircraft in 1994:
They are often associated with bluish white tendrils hanging below and arcing branches above. Despite often being categorized as such, sprites are not lightning. They are cold plasma phenomena, a bit like a fluorescent tube discharge, that are triggered by lightning in the troposphere below. The physical mechanism responsible for sprite production is still unknown but they seem to be linked to Earth's electrical field system and they may be part of every medium to large thunderstorm.
Blue Jets
Whereas sprites tend to form well above the tops of thunderstorm clouds, blue jets tend to project directly upward from them, usually as a narrow cone. These phenomena tend to form, as a result, lower in the atmosphere, often straddling the stratosphere/mesosphere boundary, as shown in this image which compares them with red sprites and lightning:
(copyright: Abestrobi (Wikipedia))
While sprites seem to be triggered by lightning strikes, blue jets appear to be more strongly associated with intense hail activity. Blue jets are believed to be the result of blue emission lines from neutral or ionized molecular (N2) nitrogen gas. Blue jets are much rarer than red sprites.
Millions of meteors enter Earth's atmosphere every day, with most of them melting or vapourizing altogether as they collide with gas molecules and atoms in the mesosphere. The vast majority of meteors are the size of a pebble or smaller, and their glow as they disintegrate is not even visible from the ground. All these meteor collisions add up to about 50 metric tons of material striking the atmosphere every day, almost all of which evaporates, leaving sparse metal layers such as sodium and potassium in the mesosphere as well as iron oxides and silica-rich nano size particles. These particles, studied using radar data and rocket-borne in situ techniques, are believed to be at extremely low densities in the mesosphere but they may be what nucleates the rare noctilucent clouds that form in this layer.
THERMOSPHERE
The thermosphere is the thickest of all the atmospheric layers, beginning between 80 and 100 km above Earth and extending to between 500 and 1000 km. Its thickness depends on solar activity. For example, it experienced an unusually severe collapse during a recent deep solar minimum in 2008-2009. This layer of atmosphere, the realm of meteors, auroras and satellites, is where solar radiation makes its first contact with Earth. When solar activity is high, UV radiation from the Sun warms the thermosphere, causing it to "puff up like a marshmallow held over a campfire." The opposite happens during low solar activity. The recent contraction, now recovering, was far more intense than the ebb in solar activity can explain, suggesting that we don't yet fully understand all the dynamics of this layer.
Named after the Greek word for heat, the thermosphere can reach temperatures as high as 1500°C during the daytime and when solar activity is at a maximum. A thermometer would not be able to record this heat, however, because the energy lost through thermal radiation would overwhelm the energy transferred from the atmospheric gas. In fact, atoms and molecules are so few and far between in this layer, that there is little to no heat transfer possible between them. This means that even though individual atoms are highly energized in the sunlight, a sensory surface could not "feel" that as heat.
Below the thermosphere, all the atmospheric gases mentioned in the preceding article are mixed together by turbulence, even in the stratosphere and mesosphere where some stratification becomes evident. In this layer, however, different gases tend to form separate layers (with little or no interaction between them) based on their atomic weights. This layer, as a whole, contains mostly molecular oxygen, molecular nitrogen, atomic oxygen, atomic nitrogen and helium gases. At the lowest level, molecular nitrogen and oxygen gases exist in much the usual percentages. Molecules of oxygen and nitrogen are relatively heavier than the other gases, however, and their atmospheric levels tend to fall off as altitude increases. Lighter atomic oxygen and nitrogen gases, and then gases such as hydrogen and helium, become relatively more abundant in the uppermost reaches of the thermosphere. This graphic explains what happens in the thermosphere:
(copyright: John Emmert/NPL from Astrobiologyonline magazine)
As you can see, much of the most energetic, and therefore deadly, radiation from the Sun, such as extreme and far UV radiation, as well as some X-ray radiation, is absorbed and blocked out by the thermosphere. As mentioned, when solar radiation is high, this layer heats up and expands. When it expands it becomes less dense. Although this layer contains on average an extremely low density of gases, changes in density must be taken into account when engineers calculate the orbits of satellites. Higher density contributes to increased drag on these fast-moving bodies and those effects must be offset occasionally by brief boosts from their onboard rockets to keep their orbits from decaying and spiraling down to Earth. The International Space Station (ISS) orbits in the thermosphere at between 320 and 380 km altitude. Here it is, docked with the space shuttle Endeavour, in this May 2011 photograph:
This was the last space shuttle docking with the ISS, and the last mission for the Endeavour.
Although the gases in this layer are stratified, they do circulate thanks to diurnal heating and cooling, creating waves and tides, not unlike ocean tides. Gas ions as well as free electrons and protons, all products of the splitting of gas molecules and atoms by extreme radiation, move along in these tides and collide with neutral gases to produce powerful electrical currents in some parts of the thermosphere. In addition to these currents, and interacting with them, are the aurorae. Aurorae (the Northern and Southern Lights) occur when solar wind, itself made up of highly energized electrons, protons and other ions, collide with ions, atoms and molecules in the thermosphere at high latitudes. The atmospheric atoms and molecules become highly energized as a result and they shed this energy by emitting light at specific wavelengths, depending on the atom or molecule that's energized. For example, excited oxygen molecules emit bright red light and nitrogen molecules emit reddish purple light. The most common colour for aurorae is green and that comes from excited oxygen atoms, as in these Southern Lights captured in 1994:
If you would like to more about how the aurorae work, please see my article called "The Northern Lights."
EXOSPHERE
This is the uppermost layer of Earth's atmosphere, beginning at around 500 km altitude. Its lower boundary with the thermosphere, called the exobase or critical level, is highly variable, depending on how expanded the thermosphere is beneath it. Here there are basically no atomic collisions, because atoms are so far apart from each other. Collisions dominate the motion of gas atoms and molecules beneath this boundary, but above it atoms are governed by ballistic motion, and with sufficient velocity they can and do escape Earth's gravity. It all depends on the velocity and trajectory. Other atoms, with the right trajectory and velocity, orbit Earth a long time, as satellite gases.
The upper boundary of the exosphere extends half the way to the Moon, about 190,000 km. It is defined as the distance where the influence of solar radiation on average atomic hydrogen velocity overcomes the gravitational pull by the Earth. In fact, the exosphere consists almost entirely of neutral hydrogen atoms, the lightest of all the atmospheric gases. Earth's magnetic field protects our atmosphere from being stripped away by solar wind. It acts like an energy collector that interacts with the solar wind material and draws energy out of it. However, Earth's magnetic field also funnels that energy and guides it into the upper atmosphere (this occurs at the poles) and allowing atoms and molecules to escape through the same funnels. There is no cause for alarm, though, because the rate of atmospheric loss through both gravitational escape and escape through the magnetic field is so low, it would take until the Sun becomes a red giant, billions of years from now, to lose appreciable atmosphere.
The exosphere is UV-visible from outer space as a geocorona, which extends to about 100,000 km. This is solar far-ultraviolet light that reflects off of neutral hydrogen atoms in the atmosphere. You cannot see it with the naked eye - Earth's atmosphere appears to end sharply as seen against black outer space:
However, the atmospheric edge looks completely different when seen using UV light instead of visible light. Below is a colour enhancement of an ultraviolet photograph of Earth, with its geocorona extending far out in all directions. Sunlight is shining from the left and the geocorona is brighter on that side. You can make out Earth as outlined by the curvature of the yellow area:
The exosphere plays an important role in the plasma budget of Earth's magnetosphere. It acts as a sink for charged particles, and there is a great influx of them during geomagnetic storms. These charged particles can exchange energy with exospheric neutral hydrogen, allowing them to return to their ground states, removing plasma and restoring the exosphere to its pre-storm state.
IONOSPHERE
Another layer called the ionosphere overlaps part of the mesosphere, thermosphere and exosphere, as shown here:
VLF (very low frequency) radio waves can be used to monitor sudden ionic disturbances in the atmosphere. In the daytime, these longer wavelengths dependably bounce off the D layer (image below right). Here, unusual reception patterns can be used to observe the way the ionosphere has been affected by X-ray flares from the Sun. In this way, Sun activity can be monitored. At might, when the D layer disappears, they are reflected by higher E and F layers. When this occurs, VLF wave propagation is strongly affected by ionospheric disturbances, leading to signal variations that are large enough to make monitoring sudden ionic disturbances impossible. This graphic compares daytime and nighttime VLF propagation modes:
(graphic from the SID Monitoring Station website)
The structure of the ionosphere is distributed by gravity waves so the reflective surfaces of ionization can be wavy. This is why ham signals tend to fade in and out and sound fluttery. Gravity waves are an interesting phenomenon. Any fluid, such as water or air, can generate gravity waves when a trigger like an updraft causes a pocket of air to be displaced vertically in stable air. Because of momentum it will overshoot its rise, and then overshoot its subsequent sinking. This motion sets up a series of waves as the air tries to regain equilibrium, as shown here:
This phenomenon also happens with the charged ions of the ionosphere, which also act like a fluid.
During an intense solar flare, hard X-rays from the Sun can reach down into the D layer, greatly increase radio wave absorption and cause radio blackout. Geomagnetic storms can fragment and even temporarily destroy much of the F layer altogether. When this happens, radio transmission becomes sporadic and unpredictable. Ham, ground to air and ship to shore communications are usually affected. During intense geomagnetic storms, communications satellites can be damaged by the influx of plasma higher up in the thermosphere causing disruptions to telephone, TV, GPS and Internet service.
This is the layer of atmosphere that is ionized by solar radiation and forms the inner edge of the magnetosphere. It is a shell of electrons, charged atoms and charged molecules, a ring of plasma in other words, that stretches from about 50 km altitude all the way to the edge of the exosphere, 1000 km. These particles are charged, or ionized, by (mostly) UV radiation from the Sun. In the thermosphere, free electrons can exist for some time before they are captured by a positive ion. It is these electrons that affect radio propagation. And it is this plasma ring that becomes energized during geomagnetic storms. UV, and other kinds of sufficiently energetic radiation can dislodge an electron from a gas atom or molecule, ionizing it. The electron that is released has a great deal of energy and this is the energy that contributes to the high temperatures found in the thermosphere, for example. Free electrons are eventually captured by positive ions, a reverse of the ionization process. At lower levels in the atmosphere, this recombination process prevails because gas molecules, atoms and ions are much closer together. The outer layers tend to remain ionized. The ring current, and Earth's magnetosphere as a whole, forms a complex relationship between Earth's atmosphere and solar activity. This diagram gives you an idea of where the ring current is situated with respect to Earth:
This animation shows how Earth's magnetic field interacts with the solar wind (click on it):
The outermost white lines (magnetic field lines) in this image correspond to the outer capsule (the magnetopause) in the image above this one.
Increased solar wind from a solar storm temporarily compresses the magnetosphere. At this point the thermosphere puffs up and becomes less dense. Eventually the tailward magnetic field lines (all shown as white lines extending outward on the night side of Earth) collapse and Earth's magnetosphere recovers, with the help of the ionization sink of the exosphere.
Now that we are familiar with Earth's atmosphere, we can compare its dynamics with those of other planets, next, in Earth's Atmosphere Part 4.
Labels:
Atmosphere Series
Subscribe to:
Posts (Atom)