This pie chart shows the relative amounts of these gases:
(Image courtesy ux1.eiu.edu)
Nitrogen
Most of Earth's atmosphere is composed of nitrogen gas (78%), a colourless odourless and mostly inert, or nonreactive, gas. Although we do not need nitrogen to breathe, it is essential for life on Earth. The nitrogen cycle takes mostly unusable nitrogen gas from the air and stabilizes it in compounds that plants use for photosynthesis and growth. This vital job, called nitrogen fixation, is carried out by special bacteria in the soil.
(image courtesy of EPA, Environmental Protection Agency, U.S.)
Plants are the foundation of almost every one of Earth's food webs. They provide food for all other organisms. If you are unsure what a food web is, take a look at this 3-minute video:
We obtain the nitrogen we need by eating plants and other animals. It makes up a significant part of our bodies, as part of the building blocks of our proteins and nucleic acids.
Nitrogen's Dark Side: The Bends
We breathe in (mostly) nitrogen with every breath and it is harmless. Deep sea divers, on the other hand, know that it can be potentially fatal under certain circumstances. At depths of about 30 metres or more, the pressure of the air breathed from a SCUBA tank is about four times higher than air pressure on land. It has to have the same pressure as the surrounding water, or air would not come out of the tank. Normally no nitrogen will dissolve into the blood in your lungs, but when it is under pressure at this depth, some nitrogen will pass from the air and dissolve in your blood. This is the same principle behind making carbonated sodas. The soda is exposed to carbon dioxide gas under pressure and carbon dioxide becomes dissolved in the drink. Under a tight cap, it remains dissolved but once the cap is removed and the pressure is released, the soda fizzes as carbon dioxide escapes in tiny bubbles. If a SCUBA diver ascends too quickly, the nitrogen gas dissolved in his blood will, just like the soda, escape out of his blood into his body tissues, especially, and very painfully, into joints, hence the name "the bends." Bubbles of gas in the bloodstream are always dangerous because they can potentially block the blood flow in an artery and precipitate a stroke or heart attack. The bends, or decompression sickness as it is technically called, can be prevented by slowing ascension enough to prevent the excess formation of nitrogen bubbles. Another way to prevent the bends is to use a gas mixture with a different formulation than ordinary air, a common one being Nitrox, air enriched with up to 36% oxygen, thus reducing the amount of nitrogen present and thereby reducing the possibility of the bends, shown here:
This mixture comes with its own risk, however. If it is used below the maximum recommended depth (28 metres for 36%), the increased oxygen level can lead to oxygen toxicity, a dangerous condition we will explore a bit later.
Oxygen
21% of our atmosphere is made up of oxygen, a very pale blue odourless gas. Unlike nitrogen, oxygen is highly reactive and easily forms compounds with other elements. Although oxygen is very abundant as an element (it makes up almost half of Earth's crust by weight), it is too reactive to exist for long as either a molecular (O2) or an atomic (O) gas in our atmosphere. Its level is maintained through the photosynthesis of billions of plants, all pumping out oxygen gas as a by-product of a reaction between carbon dioxide and water to create sugars that plants use for energy and growth. The reactions involved in photosynthesis take place inside chloroplasts, special organelles within plant cells:
(Image courtesy terra.dadeschools.net)
Every green part of every plant contains chloroplasts that are filled with green pigments called chlorophyll. Chlorophyll, one of Nature's most brilliant inventions, absorbs sunlight in the blue and red parts of the spectrum and transfers that energy to the what is called the reaction centre of the chloroplast, a complex of pigments, proteins and enzymes that turn sunlight into the chemical energy that is stored in sugar molecules.
If all the plants on Earth suddenly disappeared, oxygen gas would eventually be reabsorbed into Earth's rocky crust through the chemical process of oxidation. The ten most common compounds in Earth's crust are all oxides, such as silicon dioxide and iron oxide for example, in which oxygen is chemically bound up. Some experts estimate it would take only about 5000 years to completely resorb all of Earth's atmospheric oxygen.
We, and all respiring organisms, need oxygen to live. When we breathe in air, alveoli in our lungs exchange carbon dioxide for oxygen, as shown here in this diagram:
(Copyright: helix84(Wikipedia)
Our red blood cells transport oxygen to our cells where it reacts with glucose (an energy-rich sugar) to create ATP (adenosine triphosphate). ATP is an incredibly efficient energy storage molecule that drives all metabolic processes of life, from the simplest bacteria to plants to animals, with water and carbon dioxide as by-products. Almost all animals, from simple unicellular protozoa to humans, use oxygen in this process, called cellular respiration. Some very primitive organisms, however, live in oxygen-free environments and obtain their energy from fermentation or chemical reactions that use sulfates, nitrates or sulfur as an oxidizing agent rather than oxygen. Many of these simple organisms play essential roles in the nitrogen, sulfur and carbon cycles that make life possible on Earth.
Although oxygen is essential to almost all life on Earth, there is such a thing as too much of a good thing. Too much oxygen, a concern for SCUBA divers, and a possibility for those on high supplemental oxygen (usually O2 under pressure) such as premature babies and people undergoing hyperbaric oxygen therapy, can lead to cell damage and death, with symptoms most often developing in the central nervous system, lungs and eyes. This damage occurs through a condition called oxidative damage, in which too much oxygen in cells overcomes their ability to detoxify the highly reactive intermediate molecules produced during metabolic activities. Too many peroxides and free radicals, for example, can soon overcome a cell and damage or kill it. Oxidative stress underlies some of the damage associated with many diseases including atherosclerosis and heart failure. Under strictly controlled conditions, however, reactive oxygen-based molecules can be beneficial. Our immune systems use them to kill off pathogens.
Argon
Argon, making up about 1% of the atmosphere, is a colourless odourless noble gas and that means that it is almost completely nonreactive. Its name derives from a Greek word that means "lazy one." Because it is so nonreactive it makes an efficient thermal insulation material between glass panes in energy efficient windows. It is also used to put out fires at web server farms, where it will not damage sensitive electronic equipment. Argon does not take part in any known biological processes on Earth.
Carbon Dioxide
Carbon dioxide (CO2) is so familiar to us, both as a natural byproduct of respiration and as a much-maligned pollutant and greenhouse gas, that you might be surprised to learn that it comprises just a mere 0.04% of our atmosphere.
It is part of the carbon cycle and of photosynthesis, two processes essential to life on Earth.
The basic carbon cycle is illustrated in this diagram:
(Copyright:physicalgeography.net)
However, carbon dioxide naturally increases as global temperatures increase, forming a positive feedback loop. A large part of this loop takes place in the oceans. The oceans are (at present) gigantic carbon sinks, which means that they absorb carbon dioxide out of the atmosphere, where it is dissolved in water as well as taken up in bicarbonate compounds. As ocean temperature rises, carbon dioxide solubility decreases, so as oceans warm, they absorb less of the gas from the atmosphere. Carbon dioxide is a greenhouse gas. This means that it traps the heat from the Sun in the atmosphere. So, as carbon dioxide builds up in the atmosphere, temperatures rise, the oceans warm, and yet more carbon dioxide is released into the atmosphere. This is the nature of a positive feedback loop, and it is an important facet of the current threat of global warming. There are other positive feedback loops at play in global warming, and we will explore them in detail in a later atmosphere article. What concerns climatologists most about feedback loops like this one is that they tend to take a system out of equilibrium and toward extremes. Life depends on the finely tuned homeostasis of our climate.
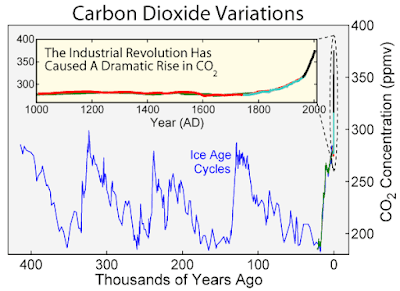
Atmospheric levels of carbon dioxide are carefully monitored because they are have been closely linked with past and present global warming events, and because Earth is currently experiencing an unprecedented spike in atmospheric CO2 levels, concurrent with industrial activity, as this graph shows:
(This figure was prepared by Robert A. Rohde from publicly available data and is incorporated into the Global Warming Art project and is subject to his copyright (Wikipedia))
Each of us produces about a kilogram of carbon dioxide every day. For us and all other animals, it is a waste gas. Yet, carbon dioxide also has an important role to play in our physiology. We have special carbon dioxide receptors in our circulatory system. If levels are high, the capillaries, tiny blood vessels where gas exchange in our tissues occurs, expand an allow more blood to the tissue. Breathing, too, is stimulated by high carbon dioxide levels, rather than by oxygen levels, in the blood. That is why low pressure air does not trigger air hunger. This is always a danger for high altitude fighter pilots and why the stewardess always tells us to put on our air mask first in an emergency, because we would not know we are running out of oxygen until we passed out. There is currently some research that links SIDS (Sudden Infant Death Syndrome) with a defect in our CO2 respiration regulation system.
Trace Gases
Our atmosphere also contains on average about 1 - 4 % water vapour. Water vapour, like carbon dioxide, is a major greenhouse gas. It too operates through a positive feedback loop. Increasing water vapour levels contribute to warmer temperatures and warmer temperatures lead to higher water vapour levels. Water vapour has also been shown to amplify the greenhouse effect of other gases. Recent research suggests that atmospheric water vapour could double the warming effect of carbon dioxide.
Other trace gases, such as neon, helium, methane, krypton, hydrogen, nitrous oxide, carbon monoxide, nitrogen dioxide, ozone, xenon, sulfur dioxide and ammonia, also contribute to Earth's atmosphere. Any one of these gases makes up far less than 1% of our atmosphere. All of them have natural origins. Nitrogen, oxygen, carbon dioxide, methane are replenished through biological activity. Hydrogen, sulfur dioxide and ozone are replenished through photochemical reactions in our atmosphere. Some gases are replenished through radioactivity - argon and helium, for example. And some gases, such as xenon, neon and krypton, are expelled from deep within Earth's interior through volcanic activity.
This graphic gives you an idea of the relative amounts of gases in Earth's atmosphere:
(copyright: Cmglee (Wikipedia))
Percentages are expressed as composition by volume; ppm means parts per million.
Our atmosphere also contains traces of gases that come from industrial activity. Carbon dioxide, nitrous oxides, carbon monoxide and sulphur dioxide, in addition to natural sources, also come from man-made combustion reactions.
Carbon Monoxide Poisoning
You have probably heard of the dangers of running your car in your garage with the doors closed, or perhaps you have heard of whole families tragically killed by a faulty furnace. The killer is carbon monoxide (CO). This colourless odourless tasteless and extremely poisonous gas is very difficult to detect and so carbon monoxide detectors are recommended for every home. CO is a product of incomplete combustion, usually linked with insufficient oxygen (an enclosed space for example). In the presence of oxygen, carbon monoxide burns with a blue flame, producing carbon dioxide. This is why gas inspectors will check the flame of a gas stove when it is installed. Blue is good. Carbon monoxide binds with hemoglobin, preferentially over oxygen, so that the body cannot deliver oxygen to its cells. Air concentrations as low as 667 parts per million, will cause half of the body's hemoglobin to bind to carbon monoxide rather than oxygen. The body's cells asphyxiate, with symptoms ranging from a mild headache to death. Quick removal of CO from the air (opening the windows and doors or getting outside) can stop the deadly process and save lives. Removal to an environment with sufficient oxygen levels will allow the CO binding process to reverse itself. Follow-up treatment for severe cases is high-dose oxygen therapy in a hospital, where binding reversal can be hastened, to help avoid permanent damage, such as irreversible coma.
Ozone Pollution
It is important to distinguish stratospheric ozone (often called "good" ozone, which we will discuss shortly) from ozone in the lower atmosphere, that is, from the surface up to an altitude of about 2 kilometres. This surface ozone is created by industrial activity and it is a pollutant. It is used as a bleach, a deodorizer and a sterilizer and it is toxic. It is also a product of internal combustion engines and power plants. Nitrous oxides and volatile organic compounds from these activities react with oxygen, especially on hot sunny days, and form ozone as a result. It is a very corrosive gas that can damage the alveoli in your lungs, leading to respiratory infections and inflamed tissues, and aggravating conditions such as asthma. It can also destroy crops and forest vegetation. Some of the highest recorded ozone levels have occurred right here in Canada, in the Toronto area. Much of our ozone pollution drifts up from the U.S. industrial belt. This graphic shows afternoon ground-level ozone concentration for last July:
(units are parts per billion volume, calculated using Harvard GEOS-CHEM model, image courtesy World Meteorological Organization)
The USDA has done extensive research on ozone plant damage, showing it to be a serious and current threat to North American crops.
Various chlorofluorocarbons, carbon tetrachloride, and carbon tetrafluoride, also considered trace atmospheric gases, come strictly from industrial activities and, although they are very minute components of our atmosphere, their levels are increasing and they are potent atmospheric pollutants. Chlorofluorocarbons provide an excellent cautionary tale about the unexpected environmental damage human activities can sometimes cause.
Chlorofluorocarbons
You might remember these gases as Freons ®, the brand name given by their maker, Dupont. These gases were very useful as refrigerants, solvents and propellants and came into widespread use in the late 1900's. As early as the 1930's, these gases were used in refrigeration and as fire retardants, and atmospheric concentrations began to rise rapidly. In the 1970's, researchers discovered a disturbing link between these chemicals and the depletion of Earth's natural ozone layer high up in the atmosphere (the stratosphere). The ozone layer helps protect Earth's surface from deadly ultraviolet radiation, especially UV-C and UV-B radiation, which are most damaging to living things, as shown by this graph:
The ozone layer is replenished naturally through oxygen (O2) reactions. The chlorine ion in chlorofluorocarbons is released in the atmosphere by a photo-induced fission of the Cl-C bond. This ion catalyzes the conversion reaction of ozone into oxygen molecules. Ozone (O3) absorbs UV radiation much better than oxygen (O2) does, so this is why the growing holes in our ozone layer, centered about the two poles, is dangerous to life. The use of chlorofluorocarbons has been almost completely phased out as this writing (2012). Chlorofluorocarbons break down quite readily in the atmosphere. And if they are released lower in the atmosphere they break up without doing damage to the ozone layer. Just the same, the breakdown of stratospheric chlorofluorocarbons has led to much higher amounts of chlorine ions than originally predicted, and these ions remain stable in the atmosphere as they continue to weaken the ozone layer. Ozone depletion has been linked to increased rates of skin cancer, cataracts, damage to plants and reduction in plankton populations, particularly at latitudes over 35°.
You might have heard of other gases in the media that are being closely monitored for their potential link to global warming, such as methane, carbon dioxide and nitrous oxides. These are all greenhouse gases. They tend to absorb heat from the Sun and trap it in our atmosphere, much like greenhouse glass does. We will explore their potential threat in a future atmosphere article focusing on global warming and climate change.
Acid Rain
Rain is naturally mildly acidic. Carbon dioxide in the air reacts with water to form weak carbonic acid. The threat of acid rain you have probably heard about is an enhanced effect, a result of some of the gases emitted when fossil fuels are burned, particularly sulphur dioxide and nitrous oxides. Fossil fuels often contain sulphur impurities that burn and create sulphur dioxide. Sulphur dioxide reacts with water to create a weak solution of sulphuric acid. Usually, nitrogen and oxygen don't react with each other in the atmosphere. But at very high temperatures, in an engine for example, a small proportion of each gas does react, to create nitrogen oxides. These oxides react with water to create a weak solution of nitric acid. When fresh water pH drops below 5, most fish eggs won't hatch and adult fish begin to die off. Aquatic insects, as well, take a severe hit, reducing the biodiversity in the freshwater ecosystem, thereby weakening it. These systems are affected not only directly by acidic rain but by acidic runoff as well. In soils, some microbes cannot handle increased acidity. Their enzymes, essential for carrying out their metabolic functions, become denatured and they die. As well, toxic metals like aluminum are released from compounds and important minerals such as magnesium and calcium are leached away from soils when pH is lowered. Crops can be fertilized and limed to counteract increasing acidity but plants in the wilderness such as natural forests suffer from the lack of nutrients that results. Humans are generally not directly affected by acid rain but our buildings are. Limestone and marble, which contain lots of calcium carbonate, erode away under acidic conditions. This is why the inscriptions on old gravestones eventually become illegible. Metals, especially iron, steel, copper and bronze, also corrode faster under acidic conditions.
Why Is The Sky Blue?
You might think that the sky is blue because of the contribution of faintly blue oxygen gas, mentioned earlier. This is what liquid oxygen looks like, to give you an idea of its colour:
(Photo courtesy Dr. Warwick Hillier, Australian National University (Wikipedia))
The sky is blue, not because of oxygen but because of how sunlight is scattered by various gas molecules in Earth's atmosphere. Sunlight is made up of all the colours of the rainbow. When all these colours are combined, they make up pure white light. Prisms (or raindrops suspended in the sky) separate white sunlight into its colours.
Notice that the blue and violet light bends more and is made up of shorter wavelengths. This light has more energy than longer wavelength red light.
When sunlight reaches Earth's atmosphere, gas molecules scatter it in all directions. Higher energy (blue) sunlight tends to scatter more than lower energy (red) sunlight. This process is called Rayleigh scattering. The sky looks blue because we see many of these scattered blue rays. Rayleigh scattering gives Earth a blue halo that is even visible from outer space, as shown in this photo taken by the International Space Station. The moon as a crescent is visible through the blue haze.:
When the Sun gets low on the horizon, sunlight must pass through even more air before it reaches our eyes. Blue light rays are scattered and rescattered many times in many directions. The surface of the Earth has also reflected and scattered the sunlight. As a result, we tend to see more white light and less blue light. As the Sun sets, even more blue light is scattered while longer wave reds and yellows are less scattered, so more of these coloured rays can travel straight to your eyes. Particulate pollution, dust and water vapour all scatter light and contribute to more dramatically colourful sunsets, such as this one in South Africa:
(copyright: Geraldbrowne (Wikipedia))
Now that we are familiar with the components that make up Earth's atmosphere, let's explore its structure, next, in Earth's Atmosphere Part 3.














No comments:
Post a Comment
Note: Only a member of this blog may post a comment.